Abstract
Wastewater-based epidemiology (WBE) for quantification of illicit drug biomarkers (IDBs) in wastewater samples is an effective tool that can provide information about drug consumption. The most commonly quantified IDBs belong to different chemical classes, including cocaine, amphetamine-type stimulants, opioids, and cannabinoids, so the different chemical properties of these molecules pose a challenge in the development of analytical methods for multi-analyte analysis. Recent workflows include the steps of sampling and storage, sample preparation using solid-phase extraction (SPE) or without extraction, and quantification of analytes employing gas or liquid chromatography coupled with mass spectrometry. The greatest difficulty is due to the fact that wastewater samples are complex chemical mixtures containing analytes with different chemical properties, often present at low concentrations. Therefore, in the development of analytical methods, there is the need to simplify and optimize the analytical workflows, reducing associated uncertainties, analysis times, and costs. The present work provides a critical bibliographic survey of studies published from the year 2020 until now, highlighting the challenges and trends of published analytical workflows for the multi-analysis of IDBs in wastewater samples, considering sampling and sample preparation, method validation, and analytical techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumption of drugs of abuse has increased in recent years, becoming a problem on a global scale, with implications for public health, security, and the economy [1,2,3]. According to the 2022 World Drug Report, 284 million people worldwide, aged from 15 to 64 years, used a drug in 2020, representing a 26% increase over 2010 data [4]. Drug consumption leads to environmental and health concerns, because wastewater treatment plants (WWTPs) are not designed to completely remove these compounds, which are considered emerging contaminants [5,6,7,8].
Traditionally, drug consumption data are obtained using population surveys combined with crime statistics and medical reports [7, 9,10,11,12,13]. However, this approach cannot monitor rapid changes in drug use [1, 7], in addition to being expensive and time-consuming and potentially underestimating actual consumption levels due to social taboos [7, 11, 12, 14, 15]. A complementary tool that has become consolidated globally is wastewater-based epidemiology (WBE), involving the quantification of chemical or biological markers, such as illicit drugs and/or their stable metabolites, from the analysis of untreated wastewater containing these substances excreted from the human body in urine and feces [3, 9, 10, 16]. The application of WBE for drug detection allows the estimation of short- and long-term consumption levels, with the advantages of being a faster, direct (almost real-time), noninvasive, accurate, and less laborious tool, compared to the use of population surveys [1, 3, 8, 11]. It is possible to investigate temporal trends of drug use by the community [3, 16] and in educational institutions [17], the use of new psychoactive substances (NPS) [18, 19], the influence of weekends and national holidays [20], festive events [6, 20], seasons [1, 9], and music events [12, 21], and the impact of social isolation measures imposed due to crises such as the COVID-19 pandemic [5, 10, 22,23,24,25].
The use of WBE employing multi-analysis of illicit drug biomarkers (IDBs) is highly attractive, because it can reduce costs and analysis times [26]. However, a wastewater sample is a chemically complex matrix, so it is a major challenge to develop analytical methodologies for the identification and quantification of compounds with different properties, especially considering the wide range of pKa and hydrophilic/lipophilic characteristics [20]. The classic illicit drugs most investigated in WBE (see Figure S1) are represented by four chemical classes: cocaine, amphetamine-type stimulants (ATS), opioids, and cannabinoids [1, 9, 16, 22]. Cannabinoids are not always included in the same analysis with other IDBs (medium to high polarity) due to the low polarity, lipophilicity, and acidic character (pKa ≅ 4.7) of the main biomarker, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) [7, 14, 15].
Several published studies have reported different analytical workflows for the detection and quantification of IDBs in wastewater. The most common is the extraction of analytes by solid-phase extraction (SPE) followed by analysis using liquid chromatography–tandem mass spectrometry (LC–MS/MS). However, other less common analytical protocols for the quantification of IDBs have also been reported [27,28,29]. The aim of the present work is to discuss the challenges and trends of analytical workflows for multi-analysis of IDBs in wastewater samples, providing a critical appraisal of sample collection and preparation methods, validation procedures, and analytical techniques. In an attempt to capture recent trends, most of the studies cited were published from the year 2020 onwards. Table S1 summarizes the information on analytical workflows of the most recent studies concerning the identification of IDBs in wastewater.
Sampling and conditioning after collection
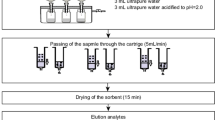
The analytical procedures in WBE for IDBs involve the steps of sampling, sample preparation, and instrumental analysis [11, 26], shown graphically in Fig. 1.
The sampling methodology directly affects the representativeness of the samples [30], so for this reason, 24-h composite sampling of wastewater using active sampling with autosamplers has been the predominant approach [1,2,3, 5,6,7, 11, 12, 31, 32]. Given that autosamplers are sophisticated equipment, other sampling strategies have also been used, such as short sampling times [17] and grab sampling [20, 33, 34].
Passive sampling has attracted increasing attention, especially due to its low cost [27]. The most reported passive sampling method for the determination of IDBs in wastewater is the use of polar organic chemical integrative samplers (POCIS), which consist of a medium to high hydrophilicity solid phase compressed between two porous membranes [16, 27]. These samplers can be immersed in water for more than a week, accumulating compounds by passive diffusion [16, 27, 30], enabling the preconcentration of analytes and attainment of low quantification limits [27]. While 24-h composite sampling allows monitoring of the rise and fall of drug consumption during specific days, such as at weekends, POCIS makes it possible to monitor long-term peak events [16, 30]. The study carried out by Bishop et al. [16] compared the estimates of drug use obtained by 24-h composite sampling and passive sampling using POCIS for 30 days. Although the POCIS values were mostly underestimates (possibly due to occlusion of the POCIS membranes), the results were considered acceptable. It should be noted that POCIS has limited application for cannabinoids, possibly due to the composition of the sorbent, and, to the best of our knowledge, has only been adopted in a few studies [27].
Despite presenting adequate performance, other limitations of POCIS are related to the presence of suspended solids and microorganisms, as well as the possibility of accumulation of analytes in the membrane, instead of in the adsorptive phase, which can compromise sampling performance [16]. As an alternative, McKay et al. [30] suggested two configurations of passive samplers with affinity for acidic, neutral, and basic analytes, using (1) microporous polyethylene tubes (MPTs) loaded with a hydrophilic and lipophilic sorbent, and (2) MPTs loaded with a gel formed by a sorbent with the aforementioned properties, together with agarose. The MPT sampler was considered sensitive and presented linear accumulation over time for cocaine, ATS, opioids, and 20 other compounds. Another type of passive sampling that can be highlighted is the use of diffusive gradients in thin films (o-DGT) to sample organic compounds. Liu et al. [35] tested three different resins (Oasis HLB from Waters Corporation, USA; XAD18 from Sigma-Aldrich, USA; and XDA-1 from Sunresin Co. Ltd., China) as binders to the o-DGT membrane for passive sampling of 15 illicit drugs and other organic compounds. These resins provided similar results in terms of the amounts and concentrations of the analytes quantified.
Several studies have suggested that wastewater sampling should be performed by collection proportional to the flow, which takes into account possible fluctuations in concentrations and flows over time [6, 7, 24]. However, this requires the use of a flowmeter to activate an automatic sampler, which limits its application [36]. Consequently, sampling proportional to time is most widely employed, where the recommended collection interval does not exceed 5–10 min, to follow the flux of toilet flushes [15]. In addition, an ideal monitoring campaign requires long-term sampling at a wide range of WWTPs [32], enabling the tracking of possible consumption peaks and the entry of new psychoactive drugs over time. However, due to technical limitations, the 7-day sampling campaign is the most frequent [2, 11, 21, 32, 37,38,39].
After sample collection, one of the greatest challenges in WBE is to maintain the chemical stability of IDBs, since they can be significantly biotransformed within hours. This is especially important in situations where it is not possible to analyze the samples immediately, which requires that they be stored (sometimes for weeks) under appropriate conditions [15]. Storage of raw wastewater samples at low temperature is often used to decrease microbial degradation, with temperatures of 4 ºC [6, 7, 12, 16, 23, 39, 40] and especially −20 ºC [8, 9, 32, 33, 37, 38, 41, 42] being most reported. This strategy can be effective in maintaining short-term stability. However, for long-term storage, freezing the SPE cartridge before elution has been shown to be the best way to ensure the stability of IDBs [11, 43]. In addition, adjustment of samples to pH 2 is often performed [1, 7, 16, 20, 21, 33, 41, 44, 45], which inhibits microbial activity [15, 16]. However, an acidic medium can lead to decreases of the concentrations of opioids [41] and favors the biotransformation of THC-COOH into 11-hydroxy-Δ9-tetrahydrocannabinol (THC-OH) [15], making it difficult to recover in the samples. Hence, some studies have avoided acidification [43], maintaining the pH at the value measured at the time of collection, which is usually between 7 and 8 [3, 6, 9, 10, 12, 24, 37].
Sample preparation
Sample preparation represents the most costly and time-consuming step, and possible errors can lead to sample contamination and loss of analytes, affecting the accuracy of the results. Wastewater samples collected are composed of suspended solids, colloids, and microorganisms that can adsorb the analytes or can cause clogging of the SPE cartridges, affecting the accuracy of the results [26, 41]. Centrifugation and filtration with fiber filters are the two most widely used approaches to remove these compounds [11, 39, 41, 44]. An alternative to the aforementioned filtration is the use of syringe filters before SPE [27, 33, 41] and/or before injection in the analytical instrument [6, 38, 40, 43, 45]. However, the possible loss of some IDBs during filtration processes must be considered (especially for less polar compounds, such as cannabinoids), since the compounds may be adsorbed on the material [16]. Pandopulos et al. [46] investigated the loss of cannabinoids onto a glass microfiber filter, in comparison to analysis by LC–MS/MS without filtration, observing that the filtration step significantly reduced the recoveries.
Wastewater samples are chemically complex mixtures with analytes present at very low concentrations (on the order of nanograms per liter), so the analysis requires the application of effective sample preparation methods to remove interferents and preconcentrate the target analytes, in order to provide greater sensitivity in the analytical determination of IDBs [29, 47]. SPE is the predominant sample preparation technique used in WBE research [5, 48]. Most studies have employed offline SPE [1, 6, 9, 11, 21, 23, 24, 37], where sample preparation and chromatographic analysis are performed separately [26]. However, disadvantages of this approach are that it is time-consuming, uses higher volumes of sample and solvents, and can suffer from analyte losses that decrease the extraction efficiency [41]. An alternative is to use online SPE [41] coupled with gas or liquid chromatographic analysis, which provides an automated and sequential process [15, 26], decreasing the number of steps and, consequently, reducing both the possibility of contamination and the use of organic solvents [41]. In comparison of online and offline SPE protocols, Wang et al. [41] reported variations lower than 21% in the concentrations of 12 IDBs, indicating the suitability of online SPE as a way to simplify the procedure and reduce the time required for extraction of IDBs from wastewater samples. However, limitations of online SPE are related to optimization of the elution steps and especially the preconcentration [49], where a high sample volume is required to reach detectable levels, which may not be supported by the technique.
The selection of an appropriate sorbent for SPE is crucial, since it must ensure effective extraction and recovery of the analytes, without any significant losses [1]. The type of sorbent employed must consider the properties and the chemical structures of the target IBDs [5]. Reversed-phase sorbents with hydrophilic and lipophilic properties, such as Oasis HLB and Strata-X (Phenomenex, CA, USA), have been extensively used in the multi-analysis of molecules in a wide polarity range, providing good recovery percentages in the extraction of IDBs [9, 11, 12, 31, 37, 41].
Another more selective sorbent for SPE is the mixed-mode type, with reversed-phase and strong cation exchange, such as Oasis MCX (Waters Corporation, MA, USA) and Strata-X-C (Phenomenex, CA, USA) [1, 7, 16, 17, 21, 23, 45]. Although the addition of sulfonic acid to the divinylbenzene group makes it highly selective for basic compounds, these sorbents have been found to provide good recovery percentages (around 80%) for cannabinoids, as reported in studies carried out by Sulej-Suchomska et al. [1] and Cruz-Cruz et al. [2]. Christophoridis et al. [39] compared the use of Oasis HLB and Oasis MCX cartridges for extraction of IDBs from wastewater samples, where the HLB cartridges provided slightly lower recoveries for all the compounds evaluated (except for cannabinoids). More recently, a novel mixed-mode weak cation exchange sorbent for SPE was proposed, involving the modification of polystyrene-divinylbenzene (PS-DVB) microspheres with mercaptosuccinic acid. The authors reported satisfactory recoveries (84–106%) for the extraction of cocaine, ATS, and opioids, when the sorbent was compared with other SPE cartridges (Oasis HLB, Oasis MCX, and Oasis WCX, from Waters Corporation, MA, USA) [47].
In general, the reported studies show that there is no single solution when selecting the SPE sorbent, especially when cannabinoids (low polarity) are included with other polar analytes from the classes of cocaine, ATS, and opioids. It is likely that one of the main factors leading to diverse analytical results, comparing different studies that used a similar SPE sorbent, is the strong influence of the wastewater matrix, whose chemical composition varies considerably according to region [30].
The solvents employed in SPE of IDBs in wastewater depend on the composition of the chosen cartridge, although methanol is by far the most widely used. In the cartridge conditioning step, the commonest procedure is to pass methanol followed by ultrapure water [3, 6, 9, 11, 12, 37, 39, 43], or acidified ultrapure water when a mixed-mode cation exchange sorbent (MCX) is used [1, 2, 5, 7, 16, 17, 21, 26]. The reported volumes of wastewater samples range from 50 mL to 1 L, at low flow rates, while for MCX sorbents, sample acidification may be necessary to promote ionization of the molecules [5, 26]. The cleanup step uses a washing solvent that must be sufficiently strong to remove the interferents, with the most common being water or methanol [2, 3, 9, 12, 24, 37, 41, 43]. However, a cleanup step is not always performed, since it can cause losses of analytes [1, 5, 6, 11, 17, 21]. For the elution step, methanol (sometimes with acid or base additives) is typically used [3, 6, 9, 11, 12, 32, 37, 39, 41, 43]. The use of methanol basified with ammonia is less commonly reported, but is suitable for elution from MCX sorbents [1, 7, 16, 17, 21], with the objective of neutralizing the charges and disrupting the electrostatic interactions between the analytes and the sorbent [26].
Despite being the dominant technique, drawbacks of SPE that need to be overcome include the cost and the time required. Liquid–liquid extraction (LLE), which is faster and consists of simpler steps, has been applied for the quantification of cannabinoids in wastewater samples [46, 50], considering that the other IDBs have high water solubility, which hinders their mass transfer to the organic phase. Liquid-phase microextraction (LPME) has also been applied due to its simplicity and use of lower volumes of organic solvents (μL) and sample [25]. Wu et al. [8] developed an enrichment bag-based liquid-phase microextraction (EB-LPME) technique, where a flat polypropylene membrane bag was used for the extraction of six IDBs. In order to automate LPME, Nascimento et al. [25] developed a semi-automated LPME method for the simultaneous extraction of nine illicit drugs.
Chen et al. [48] investigated the use of thin-film microextraction (TFME) with polydimethylsiloxane (PDMS) loaded with divinylbenzene (DVB) particles, which provided rapid extraction of IDBs using low volumes of sample and solvent, although the TFME efficiency was strongly influenced by pH, with low performance in an acidic medium. In another study, Zhang et al. [29] synthesized magnetic polystyrene-divinylbenzene-glycidylmethacrylate microspheres modified with nano-petal-shaped covalent organic frameworks (NP-COF@Mag-PS/DVB/GMA), applied as sorbent for magnetic dispersive SPE of 12 IDBs in wastewater samples. The authors reported that the microspheres could be reused 20 times and that the extraction efficiency was dependent on the pH of the sample (~ 6–8), since acidic and basic conditions could suppress the hydrogen bonds between the –NH– groups in the IDBs and the microspheres due to the ionic state of the polar drugs.
The available information suggests that although the use of SPE for multiple classes of illicit drugs can be challenging and costly, especially in the case of large monitoring campaigns, this technique is by far the most effective extraction option due to the efficiency of preconcentration and isolation of analytes. For this reason, the commercial SPE products currently employed are reliable and have become the industry standards.
Nonetheless, other studies have employed the direct injection (DI) of wastewater samples after applying sample filtration and/or centrifugation [5, 32,33,34, 42, 44]. Ng et al. [34] developed a method employing DI into an LC–MS/MS instrument for determination of compounds belonging to the cocaine, ATS, and opioid classes, achieving fast separation and adequate sensitivity. Comparison was made of the results obtained by DI and SPE, which showed that DI was more reliable, since the areas of the illicit drug peaks were considerably larger and detectability of the compounds was higher than when using SPE. It appears that DI of wastewater into chromatography instruments may be an attractive option when the analyte concentration meets the minimum validation requirements, especially the limit of quantification (LOQ). It is important to point out that technological advances currently allow LOQ values to be obtained using DI that would previously have required sample preconcentration steps. However, it is necessary to evaluate possible strong matrix effects, and there may be a need for more regular maintenance of the analytical instruments.
Analytical Techniques
LC–MS/MS has been extensively described for the identification/quantification of IDBs in wastewater samples [3, 6, 8, 9, 12, 24, 30, 43] due to its robustness, excellent sensitivity and selectivity, and advantages such as good separation with short retention times [8, 14, 15, 27, 34]. The LC systems reported include the use of high-performance liquid chromatography (HPLC) [1, 31, 38], ultra-high-performance liquid chromatography (UHPLC) [7, 27, 37, 41, 42, 47], and ultra-performance liquid chromatography (UPLC) [11, 17, 39, 44, 45]. UPLC and UHPLC have similar configurations and are based on the separation principles of HPLC, but using a stationary phase with smaller particle diameter, which generates greater pressure in the chromatographic column, resulting in increased sensitivity and faster analysis [51]. Micro-liquid chromatography–tandem mass spectrometry (µLC-MS/MS) has also been reported for the analysis of cocaine, ATS, and NPS, with the advantage of using a low flow rate, resulting in greater ionization efficiency (less suppression of ions) and increased sensitivity of the analysis, compared to conventional UHPLC [52].
In the LC methods, the main separation mechanism reported is reversed-phase [12], with octadecyl silica (C18) as the commonest stationary phase [1, 6, 7, 27, 29, 32, 37, 39, 42]. Biphenyl [3, 21, 24, 34, 38], pentafluorophenyl [8, 9, 23], and hydrophilic interaction liquid chromatography (HILIC) [5, 53] columns have also been used due to the polar character of most IDBs. The solvents employed are frequently water as mobile phase A and methanol or acetonitrile as mobile phase B, usually with the addition of acid to favor the protonation of molecules [1]. The favoring of protonation is extremely important, considering that electrospray ionization (ESI) is the main ionization method [1,2,3, 7, 8, 10,11,12, 27,28,29], with the basic character of cocaine, ATS, and opioids favoring determination in positive acquisition mode [7, 8, 16, 21, 26, 32, 33, 41]. However, when acidic molecules (such as cannabinoids) are included among the analytes, two frequently used procedures are either to maintain positive-mode ionization for all classes of drugs [2, 3, 5, 6, 9, 24, 39] or to add negative-mode acquisition for cannabinoids [1, 3, 12, 17, 21, 23, 27, 37], since the latter provides more abundant ionization for cannabis molecules [1].
In the case of LC–MS/MS, the triple quadrupole (QqQ) is the most reported m/z analyzer [6, 7, 10, 12, 16, 31, 34, 38, 39, 41, 42] due to its sensitivity, selectivity, and robustness [14, 15, 34]. A hybrid triple quadrupole-linear ion trap (Qtrap) has also been used [17, 21, 24, 30, 33, 45, 47]. However, these analyzers may have limitations when the monitoring of many analytes is desired, due to the number of consecutive scans required for the quantification/confirmation of compounds, which makes the window time very wide. Therefore, high-resolution mass spectrometry (HRMS) has been explored as an alternative due to its potential to identify analytes by means of the exact mass [15]. For this, the Orbitrap analyzer has been most frequently cited in studies of illicit drugs in wastewater [2, 5, 24].
Despite its high sensitivity, reproducibility, and avoidance of the use of liquid solvents, gas chromatography/mass spectrometry (GC/MS) has been infrequently reported as a common analytical technique for the analysis of IDBs in wastewater, possibly due to the polar character of most of the compounds [7, 8, 14] as well as the need for derivatization with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) [54] or N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) [55] prior to analysis, which makes the procedure more laborious [15, 56]. The chromatographic run times are also longer for GC/MS, compared to LC–MS/MS. In terms of GC columns, satisfactory separations of target analytes have been obtained using 5%-phenyl methyl siloxane columns, as in the work of Cong et al. [54], who quantified cocaine, ATS, and opioid compounds in wastewater samples using offline SPE and GC/MS analysis. The gas chromatograph was coupled to a mass spectrometer operating with an electron ionization (EI) source and a single quadrupole analyzer, while other work has used an ion-trap analyzer [55].
Considering that not every drug present in wastewater originates from illicit consumption, and that most IDBs are chiral (as racemates or single enantiomers) [55], enantioselective separation of chiral drugs has been explored as a complementary tool [13, 43, 53, 55]. This approach allows a better understanding of the different pathways of drug synthesis, as well as the differences between prescription drug use, illicit use, and direct dumping [13, 22]. For example, ATS have an asymmetric carbon with two enantiomers [R-(−) and S-(+)] [17], so these are the IDBs that have been most investigated using LC–MS/MS [13]. Recent studies have reported the analysis of ATS by GC/MS [13, 55], with extension to cocaine and opioids [43], but it was essential to use chiral columns (such as cellobiohydrolase phase), which are more expensive than non-chiral columns [22]. To overcome this, some studies have added chiral derivatization reagents in the organic extract obtained after SPE, such as (R)‐(−)‐α‐methoxy‐α‐(trifluoromethyl)-phenylacetyl chloride [(R)‐MTPA‐Cl], which proved to be more economical due to the use of conventional phenyl methyl siloxane columns for GC/MS analysis [13, 40, 55].
The sample preparation and instrumental analysis techniques discussed above are costly and tend to be time-consuming. As an alternative, Mao et al. [28] synthesized Au@Ag biosensors immobilized on a nanoporous glass paper functionalized with poly-L-lysine (pLL), with detection by surface-enhanced Raman spectroscopy (SERS), where the noble metal nanostructures promoted electromagnetic enhancement. Although the study was initially limited to the analysis of methamphetamine in wastewater, the results were in agreement with analysis by LC–MS/MS, showing that the method was a viable and innovative alternative to mass spectrometry. The work was restricted to one analyte, so the viability of the method for use in multi-analysis of illicit drugs remains unproven. However, it is expected that studies employing biosensors will be extended to other IDBs as an alternative to SPE followed by LC–MS/MS or GC/MS analysis.
Analytical validation and consumption estimates
The main objective of analytical workflows is to develop methods that can provide reliable results and quantification of all the target analytes, with low limits of detection (LOD) and quantification (LOQ) [15]. To the best of our knowledge, there are no specific guidelines available concerning analytical validation of WBE methods for quantification of IDBs, although recent studies have used protocols provided by the International Conference on Harmonization (ICH) [3, 34, 40, 55], the European Medicines Agency (EMA) [7, 53], the International Union of Pure and Applied Chemistry (IUPAC) [25, 37], the Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry [29, 47, 57], and the Scientific Working Group for Forensic Toxicology (SWGTOX) [37]. It is also worth mentioning the best-practice protocol developed by the European Monitoring Center for Drugs and Drug Addiction (EMCDDA) and the Sewage analysis CORe group—Europe (SCORE) [58], as well as the established analytical methodology developed by Castiglioni et al. [59]. In general, the validation protocols employ figures of merit including selectivity, linearity, LOD, LOQ, accuracy, precision, matrix effect, and recovery [1, 3, 16, 21, 24, 37, 41].
Due to the unpredictable chemical composition of wastewater samples, it is a challenge to control the influence of matrix effects on the ionization of analytes in LC–MS/MS or GC/MS [8, 25]. Among the main strategies used to correct for these effects, the most successful has been the addition of deuterated internal standards (IS) to the samples [1, 11, 16, 25], as reported in all 30 of the studies considered in Tables S1 and S2. The use of IS can also be effective in correcting for possible losses of analytes during extraction and instrumental analysis [11, 16, 31]. Method validation guidelines indicate that acceptable recovery values for IDBs in wastewater are in the range from 80 to 120% [40]. However, it is evident that in multi-analysis approaches, the diversity of chemical compounds, with most IDBs being polar compounds, makes it difficult to meet the recovery requirements for the quantification of THC-COOH [46, 50] due to its low polarity and lipophilic character.
The LOD and LOQ values are defined as the lowest detected and quantified concentrations of analytes present in a sample, respectively. The most traditional method for LOD and LOQ calculation employs the values obtained for replicates of analytical blanks, although some authors have used administrative values [16, 21, 24, 37, 41]. However, obtaining analytes-free samples represents one of the major problems faced in the validation of analytical methods for the determination of IDBs in wastewater. Hence, water or the mobile phase have often been used [20, 38].
The SWGTOX guideline indicates that a laboratory can define analyte LOQ values according to an administrative decision, even when lower LOQ values might be obtained using replicates of analytical blanks [60]. Table S2 summarizes the LOD and LOQ values of the 30 most recent studies concerning the identification of IDBs in wastewater. It can be seen that lower LOD and LOQ values were achieved using HPLC, UPLC, or UHPLC coupled to tandem mass spectrometry with ESI ionization. These instrumental systems enabled low LOD and LOQ values to be obtained in the studies of Zhao et al. [38] and Sulej Suchomska et al. [1], mainly using UPLC and UHPLC, where a small particle diameter of the stationary phase provided better peak resolution [41, 45]. It can also be seen in Table S2 that the use of GC/MS instrumentation resulted in low LOD and LOQ values [54], which were very similar to those obtained using LC instrumentation.
LOD and LOQ values ≤ 5 ng L−1 were obtained using offline SPE [1, 38, 45, 54, 61] or online SPE [41], showing the excellent ability of SPE protocols to preconcentrate analytes and provide sample cleanup. Alternative methods using EB-LPME for the extraction of ATS and opioids also achieved low LOD and LOQ (around 10 ng L−1) [8]. Higher LOD and LOQ values were found by Nascimento et al. [25], who employed LPME and GC/MS for quantification of cocaine and ATS, which could be explained by the absence of the derivatization step required for analysis of polar IDB compounds. It is noteworthy that the highest LOD and LOQ values shown in Table S2 were obtained in a study using MPT passive sampling and LC–MS/MS analysis [30], where sample preparation was performed without a cleanup step, so the matrix effect was likely to affect the performance of the analysis, because ion suppression in the ESI could affect the IDB signal responses.
Comparison of methods that used an extraction step [1, 38, 45] with techniques based on DI of the samples [33, 34, 42] (Table S2) showed that the LOD and LOQ values were higher without an extraction step, which could be explained by matrix effects and the absence of the preconcentration factor inherent in extraction techniques such as SPE. In the work of Cruz-Cruz et al. [2], employing LC-HRMS analysis, higher LOD and LOQ values for cocaine, ATS, opioids, and cannabinoids were obtained, when compared to the LC methods using tandem mass spectrometry with low-resolution m/z analyzers. This could be explained by the lower sensitivity of the HRMS technique, where the exact masses of ions were obtained but without selected ion monitoring.
Finally, a goal of WBE studies is to perform back-calculations from the quantified IDB values in order to estimate per capita consumption of drugs, as shown in the equation below. For this, it is necessary to have knowledge of other parameters including the concentration (ng L−1) of the biomarker (C), the daily wastewater flow (Qv), a correction factor (f) based on the molar mass ratio between the parent compound/metabolite and its percentage excretion in urine, and the population served by the WWTP (inhab) [59, 62, 63].
For example, Sodré et al. [6] used this equation to obtain average daily consumption values for cocaine and cannabis in Brazil, during a typical week (no festive events), of 1739 and 11,471 mg/1000inhab/day, respectively. These values compared with 2754 and 14,342 mg/1000inhab/day for cocaine and cannabis, respectively, during a festive carnival period. Hence, the consumption estimates indicated an increase in the per capita consumption of drugs during festive events, which could provide useful information for public safety actions.
The population served by the WWTP can be estimated using census data or chemical parameters that make it possible to monitor population fluctuations due to holidays and festive events [3]. In recent years, the most widely used parameters have been biological oxygen demand (BOD) [5, 16], chemical oxygen demand (COD) [10, 12], ammonia (product of urea hydrolysis) [11, 21, 54], and creatine concentration [20]. Although reported in recent studies, the use of COD is not recommended as an indicator of population size [64], with NH4-N [3] and total nitrogen [24] being considered the most reliable and realistic parameters. The decision concerning which to use will also strongly depend on equipment availability and the analytical costs [24].
Outlook
It is evident that WBE studies represent an important way to estimate the consumption of drugs of abuse by a given community or to monitor their use during festive events and holidays, providing essential information for the formulation of public security, health, and social policies. However, WBE for quantification of IDBs is a dynamic field with different analytical workflows, which, despite being effective, require further improvements in terms of efficiency, speed, and cost reduction. Advances in knowledge about the stability of analytes in wastewater samples are still required, especially for multiclass analysis, due to the difficulties inherent in storing large amounts of samples for long periods, occupying little space and with retardation of microbial degradation, which emphasizes the need for faster analytical workflows or more effective analyte preservation procedures. The main analytical challenges are associated with the chemical diversity of the IDBs, requiring the development of new sorbents for cartridges used in SPE (the most common extraction method) or other sample preparation methods that comply with the need for preconcentration and reduction of matrix effects. The increasing use of different drugs and the emergence of new psychoactive compounds require the development of novel methodologies for estimation of long-term drug consumption that are simpler, faster, and inexpensive, and can increase the reliability of results.
References
Sulej-Suchomska AM, Klupczynska A, Dereziński P, Matysiak J, Przybyłowski P, Kokot ZJ. Urban wastewater analysis as an effective tool for monitoring illegal drugs, including new psychoactive substances, in the Eastern European region. Sci Rep. 2020;10:81–7. https://doi.org/10.1038/s41598-020-61628-5.
Cruz-Cruz C, Yargeau V, Vidaña-Perez D, Schilmann A, Pineda MA, Lobato M, Hernández-Avila M, Villatoro JA, Barrientos-Gutierrez T. Opioids, stimulants, and depressant drugs in fifteen Mexican Cities: A wastewater-based epidemiological study. Int J Drug Policy. 2021;88:103027. https://doi.org/10.1016/j.drugpo.2020.103027.
Asicioglu F, KulogluGenc M, Tekin Bulbul T, Yayla M, Simsek SZ, Adioren C, Mercan S. Investigation of temporal illicit drugs, alcohol and tobacco trends in Istanbul city: Wastewater analysis of 14 treatment plants. Water Res. 2021;190:116729. https://doi.org/10.1016/j.watres.2020.116729.
United Nations Office on Drugs and Crime (ONUDC). Global overview: drug demand. 2022. Available at https://www.unodc.org/unodc/en/data-and-analysis/wdr-2022_booklet-2.html. Accessed 05 Feb 2023.
Perkons I, Tomsone LE, Sukajeva V, Neilands R, Kokina K, Pugajeva I. Qualitative fingerprinting of psychoactive pharmaceuticals, illicit drugs, and related human metabolites in wastewater: A year-long study from Riga Latvia. J Environ Chem Eng. 2022;10:108110. https://doi.org/10.1016/j.jece.2022.108110.
Sodré FF, de Freire D, JS, Alcântara DB, Maldaner AO,. Understanding Illicit Drug Use Trends During the Carnival Holiday in the Brazilian Capital Through Wastewater Analysis. Front Anal Sci. 2022;2:1–12. https://doi.org/10.3389/frans.2022.930480.
Haalck I, Löffler P, Baduel C, Wiberg K, Ahrens L, Lai FY. Mining chemical information in Swedish wastewaters for simultaneous assessment of population consumption, treatment efficiency and environmental discharge of illicit drugs. Sci Rep. 2021;11:1–14. https://doi.org/10.1038/s41598-021-92915-4.
Wu S, Dong Y, Lin B, Shen X, Xiang P, Huang C. Sensitive determination of illicit drugs in wastewater using enrichment bag-based liquid-phase microextraction and liquid-chromatography tandem mass spectrometry. J Chromatogr A. 2022;1661:462684. https://doi.org/10.1016/j.chroma.2021.462684.
Daglioglu N, Guzel EY, Atasoy A, Gören İE. Comparison of community illicit drug use in 11 cities of Turkey through wastewater-based epidemiology. Environ Sci Pollut Res. 2021;28:15076–89. https://doi.org/10.1007/s11356-020-11404-9.
Hahn RZ, Bastiani MF, Lizot LDLF, Schneider A, Moreira ICS, Meireles YF, Viana MF, Nascimento CA, Linden R. Long-term monitoring of drug consumption patterns during the COVID-19 pandemic in a small-sized community in Brazil through wastewater-based epidemiology. Chemosphere. 2022;302: 134907.
Croft TL, Huffines RA, Pathak M, Subedi B (2020) Prevalence of illicit and prescribed neuropsychiatric drugs in three communities in Kentucky using wastewater-based epidemiology and Monte Carlo simulation for the estimation of associated uncertainties. J Hazard Mater. 384. https://doi.org/10.1016/j.jhazmat.2019.121306
Devault DA, Peyré A, Jaupitre O, Daveluy A, Karolak S (2020) The effect of the Music Day event on community drug use. Forensic Sci Int. 309. https://doi.org/10.1016/j.forsciint.2020.110226
Verovšek T, Heath D, Heath E (2022) Enantiomeric profiling of amphetamines in wastewater using chiral derivatisation with gas chromatographic-tandem mass spectrometric detection. Sci Total Environ 835:0–4. https://doi.org/10.1016/j.scitotenv.2022.155594
Huizer M, ter Laak TL, de Voogt P, van Wezel AP. Wastewater-based epidemiology for illicit drugs: A critical review on global data. Water Res. 2021;207:117789. https://doi.org/10.1016/j.watres.2021.117789.
Hernández F, Castiglioni S, Covaci A, de Voogt P, Emke E, Kasprzyk-Hordern B, Ort C, Reid M, Sancho JV, Thomas KV, van Nuijs ALN, Zuccato E, Bijlsma L. Mass spectrometric strategies for the investigation of biomarkers of illicit drug use in wastewater. Mass Spectrom Rev. 2018;37:258–80. https://doi.org/10.1002/mas.21525.
Bishop N, Jones-Lepp T, Margetts M, Sykes J, Alvarez D, Keil DE. Wastewater-based epidemiology pilot study to examine drug use in the Western United States. Sci Total Environ. 2020;745:140697. https://doi.org/10.1016/j.scitotenv.2020.140697.
Verovšek T, Krizman-Matasic I, Heath D, Heath E (2021) Investigation of drugs of abuse in educational institutions using wastewater analysis. Sci Total Environ 799. https://doi.org/10.1016/j.scitotenv.2021.150013
Bade R, White JM, Nguyen L, Tscharke BJ, Mueller JF, O’Brien JW, Thomas KV, Gerber C. Determining changes in new psychoactive substance use in Australia by wastewater analysis. Sci Total Environ. 2020;731:139209. https://doi.org/10.1016/j.scitotenv.2020.139209.
Castiglioni S, Salgueiro-González N, Bijlsma L, Celma A, Gracia-Lor E, Beldean-Galea MS, Mackuľak T, Emke E, Heath E, Kasprzyk-Hordern B, Petkovic A, Poretti F, Rangelov J, Santos MM, Sremački M, Styszko K, Hernández F, Zuccato E. New psychoactive substances in several European populations assessed by wastewater-based epidemiology. Water Res. 2021;195:116983. https://doi.org/10.1016/j.watres.2021.116983.
Centazzo N, Frederick BM, Jacox A, Cheng SY, Concheiro-Guisan M. Wastewater analysis for nicotine, cocaine, amphetamines, opioids and cannabis in New York City. Forensic Sci Res. 2019;4:152–67. https://doi.org/10.1080/20961790.2019.1609388.
Benaglia L, Udrisard R, Bannwarth A, Gibson A, Béen F, Lai FY, Esseiva P, Delémont O. Testing wastewater from a music festival in Switzerland to assess illicit drug use. Forensic Sci Int. 2020;309:1–8. https://doi.org/10.1016/j.forsciint.2020.110148.
Estévez-Danta A, Bijlsma L, Capela R, Cela R, Celma A, Hernández F, Lertxundi U, Matias J, Montes R, Orive G, Prieto A, Santos MM, Rodil R, Quintana JB. Use of illicit drugs, alcohol and tobacco in Spain and Portugal during the COVID-19 crisis in 2020 as measured by wastewater-based epidemiology. Sci Total Environ. 2022;836:155697. https://doi.org/10.1016/j.scitotenv.2022.155697.
Bade R, Tscharke BJ, O’Brien JW, Magsarjav S, Humphries M, Ghetia M, Thomas KV, Mueller JF, White JM, Gerber C. Impact of COVID-19 Controls on the Use of Illicit Drugs and Alcohol in Australia. Environ Sci Technol Lett. 2021;8:799–804. https://doi.org/10.1021/acs.estlett.1c00532.
Reinstadler V, Ausweger V, Grabher AL, Kreidl M, Huber S, Grander J, Haslacher S, Singer K, Schlapp-Hackl M, Sorg M, Erber H, Oberacher H. Monitoring drug consumption in Innsbruck during coronavirus disease 2019 (COVID-19) lockdown by wastewater analysis. Sci Total Environ. 2021;757:144006. https://doi.org/10.1016/j.scitotenv.2020.144006.
Nascimento MM, Nascimento ML, Anjos JF, Cunha RL, Rocha GO, Santos IF, Pereira PAP, Andrade JB. A novel method for the determination of illicit drugs in wastewater and surface wasters based on a new semi-automated liquid-liquid microextraction devide. 2022. https://doi.org/10.2139/ssrn.4246839.
Sadutto D, Picó Y (2020) Sample Preparation to Determine Pharmaceutical and Personal Care Products in an All-Water Matrix: Solid Phase Extraction. Molecules 25. https://doi.org/10.3390/molecules25215204
Hahn RZ, Bastiani MF, de Lima FeltracoLizot L, da Silva Moreira IC, Meireles YF, Schneider A, do Nascimento CA, Linden R. Determination of a comprehensive set of drugs of abuse, metabolites and human biomarkers in wastewater using passive sampling followed by UHPLC-MS/MS analysis. Microchem J. 2022;172:106960. https://doi.org/10.1016/j.microc.2021.106960.
Mao K, Zhang H, Pan Y, Zhang K, Cao H, Li X, Yang Z. Nanomaterial-based aptamer sensors for analysis of illicit drugs and evaluation of drugs consumption for wastewater-based epidemiology. TrAC - Trends Anal Chem. 2020;130:115975. https://doi.org/10.1016/j.trac.2020.115975.
Zhang S, Cui HR, Zhao YG, Zhan PP. Preparation and application of nano petal-shaped covalent organic frameworks modified polystyrene-divinylbenzene- glycidylmethacrylate microspheres for the extraction of illicit drugs from wastewater. J Chromatogr A. 2022;1682:463505. https://doi.org/10.1016/j.chroma.2022.463505.
McKay S, Tscharke B, Hawker D, Thompson K, O’Brien J, Mueller JF, Kaserzon S. Calibration and validation of a microporous polyethylene passive sampler for quantitative estimation of illicit drug and pharmaceutical and personal care product (PPCP) concentrations in wastewater influent. Sci Total Environ. 2020;704:135891. https://doi.org/10.1016/j.scitotenv.2019.135891.
Ferreira AP, Cruz-Hernández MJ. Plantas de tratamiento de aguas residuales: estimación del consumo de drogas ilícitas en la zona oeste, Rio de Janeiro. Rev Eletrônica Acervo Saúde. 2022;15:e9427. https://doi.org/10.25248/reas.e9427.2022.
ter Laak TL, Emke E, Benschop A, Nabben T, Béen F (2021) Triangulating Amsterdam’s illicit stimulant use trends by wastewater analysis and recreational drug use monitoring Thomas. J Alloys Compd 165187. https://doi.org/10.1016/j.forsciint.2022.111449
Hue TTT, Zheng Q, Anh NTK, Binh VN, Trung NQ, Trang HT, Chinh PQ, Minh LQ, Thai PK. Prevalence of illicit drug consumption in a population of Hanoi: an estimation using wastewater-based epidemiology. Sci Total Environ. 2022;815:152724. https://doi.org/10.1016/j.scitotenv.2021.152724.
Ng KT, Rapp-Wright H, Egli M, Hartmann A, Steele JC, Sosa-Hernández JE, Melchor-Martínez EM, Jacobs M, White B, Regan F, Parra-Saldivar R, Couchman L, Halden RU, Barron LP. High-throughput multi-residue quantification of contaminants of emerging concern in wastewaters enabled using direct injection liquid chromatography-tandem mass spectrometry. J Hazard Mater. 2020;398:122933. https://doi.org/10.1016/j.jhazmat.2020.122933.
Liu X, Zhang R, Cheng H, Khorram MS, Zhao S, Tham TT, Tran TM, Minh TB, Jiang B, Jin B, Zhang G. Field evaluation of diffusive gradients in thin-film passive samplers for wastewater-based epidemiology. Sci Total Environ. 2021;773:145480. https://doi.org/10.1016/j.scitotenv.2021.145480.
Causanilles A, Ruepert C, Ibáñez M, Emke E, Hernández F, de Voogt P. Occurrence and fate of illicit drugs and pharmaceuticals in wastewater from two wastewater treatment plants in Costa Rica. Sci Total Environ. 2017;599–600:98–107. https://doi.org/10.1016/j.scitotenv.2017.04.202.
Löve ASC, Ásgrímsson V, Ólafsdóttir K. Illicit drug use in Reykjavik by wastewater-based epidemiology. Sci Total Environ. 2022;803:149795. https://doi.org/10.1016/j.scitotenv.2021.149795.
Zhao J, Lu J, Zhao H, Yan Y, Dong H, Li W. Illicit Drugs and Their Metabolites in Urban Wastewater: Analysis, Occurrence and Consumption in China. SSRN Electron J. 2022;852:158457. https://doi.org/10.2139/ssrn.4139231.
Christophoridis C, Veloutsou, Sofia, Mitsika E, Zacharis CK, Christia C, Raikos N, Fytianos K (2021) Determination of illicit drugs and psychoactive pharmaceuticals in wastewater from the area of Thessaloniki ( Greece ) using LC – MS / MS : estimation of drug consumption. Environ Monit Assess. 1–15. https://doi.org/10.1007/s10661-021-09035-9
Langa I, Tiritan ME, Silva D, Ribeiro C (2021) Gas chromatography multiresidue method for enantiomeric fraction determination of psychoactive substances in effluents and river surface waters. Chemosensors 9. https://doi.org/10.3390/chemosensors9080224
Wang J, Qi L, Hou C, Zhang T, Chen M, Meng H, Su M, Xu H, Hua Z, Wang Y, Di B. Automatic analytical approach for the determination of 12 illicit drugs and nicotine metabolites in wastewater using on-line SPE-UHPLC-MS/MS. J Pharm Anal. 2021;11:739–45. https://doi.org/10.1016/j.jpha.2021.01.002.
Vieira LHR, Busetti F, Linge KL, Joll CA (2022) Development and validation of a direct Injection liquid chromatography-tandem mass spectrometry method for the analysis of illicit drugs and psychopharmaceuticals in wastewater. J Chromatogr A. 463562. https://doi.org/10.1016/j.chroma.2022.463562
Rice J, Kannan AM, Castrignanò E, Jagadeesan K, Kasprzyk-Hordern B. Wastewater-based epidemiology combined with local prescription analysis as a tool for temporalmonitoring of drugs trends - A UK perspective. Sci Total Environ. 2020;735:139433. https://doi.org/10.1016/j.scitotenv.2020.139433.
Ren H, Yuan S, Zheng J, Luo R, Qiang H, Duan W, Zhao Y, Xiang P (2022) Direct injection ultra-performance liquid chromatography- tandem mass spectrometry for the high-throughput determination of 11 illicit drugs and metabolites in wastewater. J Chromatogr A. 463587. https://doi.org/10.1016/j.chroma.2022.463587
Yuan S, Wang X, Wang R, Luo R, Shi Y, Shen B, Liu W, Yu Z, Xiang P. Simultaneous determination of 11 illicit drugs and metabolites in wastewater by UPLC-MS/MS. Water Sci Technol. 2020;82:1771–80. https://doi.org/10.2166/wst.2020.445.
Pandopulos AJ, Bade R, O’Brien JW, Tscharke BJ, Mueller JF, Thomas K, White JM, Gerber C. Towards an efficient method for the extraction and analysis of cannabinoids in wastewater. Talanta. 2020;217:121034. https://doi.org/10.1016/j.talanta.2020.121034.
Ning H, Fan Y, Liu H, Huang Z, Ke X, Xu Y, She Y (2022) Preparation and application of polystyrene-divinylbenzene sorbent with weak cation-exchange character for the selective extraction of illicit drugs in environmental water. J Chromatogr A. 1671. https://doi.org/10.1016/j.chroma.2022.462994
Chen X, Liu S, Jiang R, Luan T, Ouyang G. Rapid detection and speciation of illicit drugs via a thin-film microextraction approach for wastewater-based epidemiology study. Sci Total Environ. 2022;842:156888. https://doi.org/10.1016/j.scitotenv.2022.156888.
Andrade-Eiroa A, Canle M, Leroy-Cancellieri V, Cerdà V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC - Trends Anal Chem. 2016;80:641–54. https://doi.org/10.1016/j.trac.2015.08.015.
Campos-Mañas MC, Van Wichelen N, Covaci A, van Nuijs ALN, Ort C, Béen F, Castiglioni S, Hernández F, Bijlsma L. Analytical investigation of cannabis biomarkers in raw urban wastewater to refine consumption estimates. Water Res. 2022;223:119020. https://doi.org/10.1016/j.watres.2022.119020.
Maldaner L, Jardim ICSF. UHPLC Uma abordagem atual: desenvolvimentos e desafios recentes. Sci Chromatogr. 2012;4:197–207. https://doi.org/10.4322/sc.2012.014.
Celma A, Sancho JV, Salgueiro-González N, Castiglioni S, Zuccato E, Hernández F, Bijlsma L. Simultaneous determination of new psychoactive substances and illicit drugs in sewage: Potential of micro-liquid chromatography tandem mass spectrometry in wastewater-based epidemiology. J Chromatogr A. 2019;1602:300–9. https://doi.org/10.1016/j.chroma.2019.05.051.
Boogaerts T, Jurgelaitiene L, Dumitrascu C, Kasprzyk-Hordern B, Kannan A, Been F, Emke E, de Voogt P, Covaci A, van Nuijs ALN. Application of wastewater-based epidemiology to investigate stimulant drug, alcohol and tobacco use in Lithuanian communities. Sci Total Environ. 2021;777:145914. https://doi.org/10.1016/j.scitotenv.2021.145914.
Cong ZX, Shao XT, Liu SY, Pei W, Wang DG. Wastewater analysis reveals urban, suburban, and rural spatial patterns of illicit drug use in Dalian, China. Environ Sci Pollut Res. 2021;28:25503–13. https://doi.org/10.1007/s11356-021-12371-5.
Gonçalves R, Ribeiro C, Cravo S, Cunha SC, Pereira JA, Fernandes JO, Afonso C, Tiritan ME. Multi-residue method for enantioseparation of psychoactive substances and beta blockers by gas chromatography–mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2019;1125:121731. https://doi.org/10.1016/j.jchromb.2019.121731.
Picó Y, Barceló D. Identification of biomarkers in wastewater-based epidemiology: Main approaches and analytical methods. Trends Anal Chem J. 2020;145: 116465.
Foppe KS, Kujawinski EB, Duvallet C, Endo N, Erickson TB, Chai PR, Matus M. Analysis of 39 drugs and metabolites, including 8 glucuronide conjugates, in an upstream wastewater network via HPLC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2021;1176:122747. https://doi.org/10.1016/j.jchromb.2021.122747.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Wastewater analysis and drugs: a European multi-city study. 2022. Available at https://www.emcdda.europa.eu/publications/html/pods/waste-water-analysis_en. Accessed 05 Feb 2023.
Castiglioni S, Bijlsma L, Covaci A, Emke E, Hernández F, Reid M, Ort C, Thomas KV, Van Nuijs ALN, De Voogt P, Zuccato E. Evaluation of uncertainties associated with the determination of community drug use through the measurement of sewage drug biomarkers. Environ Sci Technol. 2013;47:1452–60. https://doi.org/10.1021/es302722f.
Toxicology S working group for forensic. Standard practices for method validation in forensic toxicology. J Anal Toxicol. 2013;37:452–74. https://doi.org/10.1093/jat/bkt054.
Kim KY, Oh JE. Evaluation of pharmaceutical abuse and illicit drug use in South Korea by wastewater-based epidemiology. J Hazard Mater. 2020;396:122622. https://doi.org/10.1016/j.jhazmat.2020.122622.
van Nuijs ALN, Mougel JF, Tarcomnicu I, Bervoets L, Blust R, Jorens PG, Neels H, Covaci A. Sewage epidemiology - A real-time approach to estimate the consumption of illicit drugs in Brussels, Belgium. Environ Int. 2011;37:612–21. https://doi.org/10.1016/j.envint.2010.12.006.
Zuccato E, Chiabrando C, Castiglioni S, Bagnati R, Fanelli R. Estimating community drug abuse by wastewater analysis. Environ Health Perspect. 2008;116:1027–32. https://doi.org/10.1289/ehp.11022.
Tscharke BJ, O’Brien JW, Ort C, Grant S, Gerber C, Bade R, Thai PK, Thomas KV, Mueller JF. Harnessing the Power of the Census: Characterizing Wastewater Treatment Plant Catchment Populations for Wastewater-Based Epidemiology. Environ Sci Technol. 2019;53:10303–11. https://doi.org/10.1021/acs.est.9b03447.
Acknowledgements
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE, Brazil – process number IBPG-0859-1.06/21) for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry 2023 with guest editors Zhi-Yuan Gu, Beatriz Jurado-Sánchez, Thomas H. Linz, Leandro Wang Hantao, Nongnoot Wongkaew, and Peng Wu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, A.F.B., de Melo Vieira, A. & Santos, J.M. Trends and challenges in analytical chemistry for multi-analysis of illicit drugs employing wastewater-based epidemiology. Anal Bioanal Chem 415, 3749–3758 (2023). https://doi.org/10.1007/s00216-023-04644-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04644-4