Abstract
As an emerging neurodegenerative disease, Alzheimer’s disease (AD) has become a leading cause of dementia in older adults. Visinin-like protein-1 (VILIP-1) is an increasingly used biomarker for AD besides the widely accepted Aβ1-40, Aβ1-42, and tau. However, significant variations exist in the commercial immuno-based assays for VILIP-1 quantification, underlining the necessity to establish a traceability chain. Certified reference materials (CRMs) located at the top of the traceability chain are traceability sources for relevant matrix standard materials. In this work, VILIP-1 solution CRM with a certified value and uncertainty of 39.82±1.52 μg·g−1 was developed and certified using amino acid-based isotope dilution mass spectrometry (AA-ID-MS) and sulfur-based isotope dilution inductively coupled plasma mass spectrometry (ID-ICP-MS). Certified values from both strategies showed great consistency, with traceability to SI units. Moreover, the candidate VILIP-1 CRM shows excellent homogeneity and can be stable for at least 7 days at −20°C and 12 months at −70°C. The VILIP-1 CRM developed can be used in value assignment to secondary calibrators and clinical matrix CRMs, showing prospects in early diagnosis and disease monitoring for AD.
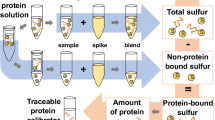
Graphical abstract








Similar content being viewed by others
References
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. The Lancet. 2021;397(10284):1577–90.
Maltsev AV, Bystryak S, Galzitskaya OV. The role of beta-amyloid peptide in neurodegenerative diseases. Ageing Res Rev. 2011;10(4):440–52.
Cecerska-Heryc E, Polikowska A, Serwin N, Roszak M, Grygorcewicz B, Heryc R, et al. Importance of oxidative stress in the pathogenesis, diagnosis, and monitoring of patients with neuropsychiatric disorders, a review. Neurochem Int. 2022;153:105269.
Li K, Wei Q, Liu FF, Hu F, Xie AJ, Zhu LQ, et al. Synaptic dysfunction in Alzheimer's disease: abeta, tau, and epigenetic alterations. Mol Neurobiol. 2018;55(4):3021–32.
El Kadmiri N, Said N, Slassi I, El Moutawakil B, Nadifi S. Biomarkers for Alzheimer disease: classical and novel candidates' review. Neuroscience. 2018;370:181–90.
Roda AR, Serra-Mir G, Montoliu-Gaya L, Tiessler L, Villegas S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer's disease. Neural Regen Res. 2022;17(8):1666–74.
Ju Y, Tam KY. Pathological mechanisms and therapeutic strategies for Alzheimer's disease. Neural Regen Res. 2022;17(3):543–9.
Dhiman K, Blennow K, Zetterberg H, Martins RN, Gupta VB. Cerebrospinal fluid biomarkers for understanding multiple aspects of Alzheimer's disease pathogenesis. Cell Mol Life Sci. 2019;76(10):1833–63.
Groblewska M, Muszynski P, Wojtulewska-Supron A, Kulczynska-Przybik A, Mroczko B. The role of visinin-like protein-1 in the pathophysiology of Alzheimer's disease. J Alzheimers Dis. 2015;47(1):17–32.
Spilker C, Dresbach T, Braunewell KH. Reversible translocation and activity-dependent localization of the calcium-myristoyl switch protein VILIP-1 to different membrane compartments in living hippocampal neurons. J Neurosci. 2002;22(17):7331–9.
Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol Neurodegener. 2009;4:20.
Babic Leko M, Borovecki F, Dejanovic N, Hof PR, Simic G. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer's disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis. 2016;50(3):765–78.
Huang S, Wang Y-J, Guo J. Biofluid biomarkers of Alzheimer’s disease: progress, problems, and perspectives. Neuroscience Bulletin. 2022;38(6):677–91.
Slany J. Directive 98/79/ec of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Journal of Developmental. 2000.
Kaan C. International Organization for Standardization (ISO). 2010:890–1.
Tarawneh R, Lee JM, Ladenson JH, Morris JC, Holtzman DM. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78(10):709–19.
Zhang H, Ng KP, Therriault J, Kang MS, Pascoal TA, Rosa-Neto P, et al. Cerebrospinal fluid phosphorylated tau, visinin-like protein-1, and chitinase-3-like protein 1 in mild cognitive impairment and Alzheimer's disease. Transl Neurodegener. 2018;7:23.
Day GS, Yarbrough MY, Körtvelyessy P, Prüss H, Bucelli RC, Fritzler MJ, et al. Prospective quantification of CSF biomarkers in antibody-mediated encephalitis. Neurology. 2021;96(20):e2546.
Villanueva J, Carrascal M, Abian J. Isotope dilution mass spectrometry for absolute quantification in proteomics: concepts and strategies. J Proteomics. 2014;96:184–99.
Feng L, Huo Z, Xiong J, Li H. Certification of amyloid-beta (abeta) certified reference materials by amino acid-based isotope dilution high-performance liquid chromatography mass spectrometry and sulfur-based high-performance liquid chromatography isotope dilution inductively coupled plasma mass spectrometry. Anal Chem. 2020;92(19):13229–37.
Arsene CG, Ohlendorf R, Burkitt W, Pritchard C, Henrion A, O'Connor G, et al. Protein quantification by isotope dilution mass spectrometry of proteolytic fragments: cleavage rate and accuracy. Anal Chem. 2008;80(11):4154–60.
Yim JH, Yoon I, Yang HJ, Kim SK, Park SR, Lee YM, et al. Quantification of recombinant human erythropoietin by amino acid analysis using isotope dilution liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(18):4401–9.
Calderon-Celis F, Encinar JR. A reflection on the role of ICP-MS in proteomics: update and future perspective. J Proteomics. 2019;198:11–7.
Wang M, Feng WY, Zhao YL, Chai ZF. ICP-MS-based strategies for protein quantification. Mass Spectrom Rev. 2010;29(2):326–48.
Feng LX, Zhang D, Wang J, Li HM. Simultaneous quantification of proteins in human serum via sulfur and iron using HPLC coupled to post-column isotope dilution mass spectrometry. Anal Methods-Uk. 2014;6(19):7655–62.
Feng L, Zhang D, Wang J, Shen D, Li H. A novel quantification strategy of transferrin and albumin in human serum by species-unspecific isotope dilution laser ablation inductively coupled plasma mass spectrometry (ICP-MS). Anal Chim Acta. 2015;884:19–25.
Lee HS, Kim SH, Jeong JS, Lee YM, Yim YH. Sulfur-based absolute quantification of proteins using isotope dilution inductively coupled plasma mass spectrometry. Metrologia. 2015;52(5):619–27.
Rodriguez-Gonzalez P, Marchante-Gayon JM, Alonso JIG, Sanz-Medel A. Isotope dilution analysis for elemental speciation: a tutorial review. Spectrochimica Acta Part B-Atomic Spectroscopy. 2005;60(2):151–207.
Acknowledgements
The authors would like to thank Ming Li, Peng Xiao, Liqing Wu, and Hui Jiao in NIM for their assistance and support with the current work.
Funding
The work was supported by the National Natural Science Foundation of China (No. 12075230) and the National Key Research and Development Program of China (No. 2017YFF0205402, No. 2021YFC2103900).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 691 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zang, Y., Zhou, X., Pan, M. et al. Certification of visinin-like protein-1 (VILIP-1) certified reference material by amino acid-based and sulfur-based liquid chromatography isotope dilution mass spectrometry. Anal Bioanal Chem 415, 211–220 (2023). https://doi.org/10.1007/s00216-022-04401-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04401-z