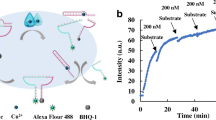
Abstract
Based on a Pb2+-specific 8–17 DNAzyme-induced catalytic hairpin assembly (CHA), a simple signal-on fluorescence strategy for lead ion detection was established. 8–17 DNAzyme was used as the recognition element of Pb2+, which catalyzed the cleavage of the RNA base embedded in the DNA substrate strand, while releasing part of the substrate strand (S’) as CHA initiator. And two hairpin probes (H1 and H2-FQ) were designed according to the sequence of S’ for CHA, in which H2-FQ was labeled with the fluorophore FAM and quencher BHQ-1 as fluorescent “molecular switch” based on fluorescence resonance energy transfer (FRET). In the presence of Pb2+, the CHA reaction was triggered to form a large number of H1-H2 complexes, enabling enzyme-free isothermal amplification and a signal-on fluorescence strategy. In the concentration range of 0.5–1000 nM, the fluorescence signal increases with the increase of Pb2+ concentration. The quantitative detection limit of Pb2+ by this method is 0.5 nM, which has better detection performance compared with the FQ-labeled 8–17 DNAzyme method. The established biosensor exhibits good specificity and can be effectively used for the detection of Pb2+ in real samples of river water and grass carp. Through ingenious nucleic acid sequence design, DNAzyme and CHA reactions are integrated to realize the enzyme-free isothermal amplifications and sensitive detection of Pb2+, which holds potential versatility in food supervision and environmental monitoring.
Graphical abstract






Similar content being viewed by others
References
Yang DX, Liu XC, Zhou YY, Lou L, Zhang JC, Huang AQ, Mao QM, Chen X, Tang L. Aptamer-based biosensors for detection of lead(II) ion: a review. Anal Methods. 2017;9(13):1976–90. https://doi.org/10.1039/c7ay00477j.
Bi XY, Li ZG, Wang SX, Zhang L, Xu R, Liu JL, Yang HM, Guo MZ. Lead isotopic compositions of selected coals, Pb/Zn ores and fuels in China and the application for source tracing. Environ Sci Technol. 2017;51(22):13502–8. https://doi.org/10.1021/acs.est.7b04119.
Huang KW, Yu CJ, Tseng WL. Sensitivity enhancement in the colorimetric detection of lead(II) ion using gallic acid-capped gold nanoparticles: improving size distribution and minimizing interparticle repulsion. Biosens Bioelectron. 2010;25(5):984–9. https://doi.org/10.1016/j.bios.2009.09.006.
Chen YY, Chang HT, Shiang YC, Hung YL, Chiang CK, Huang CC. Colorimetric assay for lead ions based on the leaching of gold nanoparticles. Anal Chem. 2009;81(22):9433v9439. https://doi.org/10.1021/ac9018268.
Steenland K, Boffetta P. Lead and cancer in humans: where are we now? Am J Ind Med. 2000;38(3):295–9. https://doi.org/10.1002/1097-0274(200009)38:3%3c295::AID-AJIM8%3e3.0.CO;2-L.
Wagner EP, Smith BW, Winefordner JD. Ultratrace determination of lead in whole blood using electrothermal atomization laser-excited atomic fluorescence spectrometry. Anal Chem. 1996;68(18):3199–203. https://doi.org/10.1021/ac9603587.
Weidenhamer JD. Circuit board analysis for lead by atomic absorption spectroscopy in a course for nonscience majors. J Chem Educ. 2007;84(7):1165–6. https://doi.org/10.1021/ed084p1165.
Qiu JY, Li ZH, Miao LJ, Wang HS, Zhang YN, Wu SS, Zhang YJ, Li X, Wu AG. Colorimetric detection of Ba2+, Cd2+ and Pb2+ based on a multifunctionalized Au NP sensor. Analyst. 2019;144(17):5081–9. https://doi.org/10.1039/c9an00836e.
Wang D, Ge CC, Lv KP, Zou QS, Liu Q, Liu LP, Yang QH, Bao SY. A simple lateral flow biosensor for rapid detection of lead(II) ions based on G-quadruplex structure-switching. Chem Commun. 2018;54(97):13718–21. https://doi.org/10.1039/c8cc06810k.
Lei YM, Huang WX, Zhao M, Chai YQ, Yuan R, Zhuo Y. Electrochemiluminescence resonance energy transfer system: mechanism and application in ratiometric aptasensor for lead ion. Anal Chem. 2015;87(15):7787–94. https://doi.org/10.1021/acs.analchem.5b01445.
Li PC, Jiang SJ. Slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry for the determination of Cr, Cd and Pb in plastics. Anal Bioanal Chem. 2006;385(6):1092–7. https://doi.org/10.1007/s00216-006-0547-6.
Zhou YY, Tang L, Zeng GM, Zhang C, Xie X, Liu YY, Wang JJ, Tang J, Zhang Y, Deng YC. Label free detection of lead using impedimetric sensor based on ordered mesoporous carbon-gold nanoparticles and DNAzyme catalytic beacons. Talanta. 2016;146:641–7. https://doi.org/10.1016/j.talanta.2015.06.063.
Liang G, Man Y, Li A, Jin XX, Liu XH, Pan LG. DNAzyme-based biosensor for detection of lead ion: a review. Microchem J. 2017;131:145–53. https://doi.org/10.1016/j.microc.2016.12.010.
Breaker RR. DNA enzymes. Nat Biotechnol. 1997;15:427–31. https://doi.org/10.1038/nbt0597-427.
Breaker RR. DNA aptamers and DNA enzymes. Curr Opin Chem Biol. 1997;1(1):26–31. https://doi.org/10.1016/S1367-5931(97)80105-6.
Khan S, Burciu B, Filipe CD, Li YF, Dellinger K, Didar TF. DNAzyme-based biosensors: immobilization strategies, applications, and future prospective. ACS Nano. 2021;15(9):13943–69. https://doi.org/10.1021/acsnano.1c04327.
Huang ZM, Wang X, Wu Z, Jian JH. Recent advances on DNAzyme-based sensing. Chem Asian J. 2022;17(6):e202101414. https://doi.org/10.1002/asia.202101414.
Carmi N, Balkhi SR, Breaker RR. Cleaving DNA with DNA. Proc Natl Acad Sci U S A. 1998;95(5):2233–7. https://doi.org/10.1073/pnas.95.5.2233.
Emilsson GM, Breaker RR. Deoxyribozymes: new activities and new applications. Cell Mol Life Sci. 2002;59(4):596–607. https://doi.org/10.1007/s00018-002-8452-4.
Zhou WH, Ding JS, Liu JW. Theranostic DNAzymes. Theranostics. 2017;7(4):1010–25. https://doi.org/10.7150/thno.17736.
Peng HY, Newbigging AM, Wang ZX, Tao J, Deng WC, Le XC, Zhang HQ. DNAzyme-mediated assays for amplified detection of nucleic acids and proteins. Anal Chem. 2018;90(1):190–207. https://doi.org/10.1021/acs.analchem.7b04926.
McConnell EM, Cozma I, Mou QB, Brennan JD, Lu Y, Li YF. Biosensing with DNAzymes. Chem Soc Rev. 2021;50(16):8954–94. https://doi.org/10.1039/d1cs00240f.
Yan L, Zhou J, Zheng Y, Gamson AS, Roembke BT, Nakayama S, Sintim HO. Isothermal amplified detection of DNA and RNA. Mol Biosyst. 2014;10(5):970–1003. https://doi.org/10.1039/c3mb70304e.
Yin P, Choi HMT, Calvert CR, Pierce NA. Programming biomolecular self-assembly pathways. Nature. 2008;451(7176):318–23. https://doi.org/10.1038/nature06451.
Li BL, Ellington AD, Chen X. Rational, modular adaptation of enzyme-free DNA circuits to multiple detection methods. Nucleic Acids Res. 2011;39(16):110. https://doi.org/10.1093/nar/gkr504.
Wang DN, Guo R, Wei YY, Zhang YZ, Zhao XY, Xu ZR. Sensitive multicolor visual detection of telomerase activity based on catalytic hairpin assembly and etching of Au nanorods. Biosens Bioelectron. 2018;122:247–53. https://doi.org/10.1016/j.bios.2018.09.064.
Liu JM, Zhang Y, Xie HB, Zhao L, Zheng L, Ye HM. Applications of catalytic hairpin assembly reaction in biosensing. Small. 2019;15(42):e1902989. https://doi.org/10.1002/smll.201902989.
Wang WH, Zhang C, Guo JX, Li GP, Ye BX, Zou LA. Sensitive electrochemical detection of oxytetracycline based on target triggered CHA and poly adenine assisted probe immobilization. Anal Chim Acta. 2021;1181:e338895. https://doi.org/10.1016/j.aca.2021.338895.
GB/T5009.268–2016 Food safety determination of multiple elements in food, National Standard of People’s Republic of China, Beijing, China.
Zhang XB, Kong RM, Lu Y. Metal ion sensors based on DNAzymes and related DNA molecules. Annu Rev Anal Chem. 2011;4(1):105–28. https://doi.org/10.1146/annurev.anchem.111808.073617.
Han ST, Zhou XH, Tang YF, He M, Zhang XY, Shi HC, Xiang Y. Practical, highly sensitive, and regenerable evanescent-wave biosensor for detection of Hg2+ and Pb2+ in water. Biosens Bioelectron. 2016;80:265–72. https://doi.org/10.1016/j.bios.2016.01.070.
Fu CC, Xu WQ, Wang HL, Ding H, Liang LJ, Cong M, Xu SP. DNAzyme-based plasmonic nanomachine for ultrasensitive selective surface-enhanced raman scattering detection of lead ions via a particle-on-a-film hot spot construction. Anal Chem. 2014;86(23):11494–7. https://doi.org/10.1021/ac5038736.
Ren W, Zhang Y, Fan YZ, Dong JX, Li NB, Luo HQ. A resonance Rayleigh scattering sensor for detection of Pb2+ ions via cleavage-induced G-wire formation. J Hazard Mater. 2017;336:195–201. https://doi.org/10.1016/j.jhazmat.2017.04.039.
Zheng J, Wai JL, Lake RJ, New SY, He ZK, Lu Y. DNAzyme sensor uses chemiluminescence resonance energy transfer for rapid, portable, and ratiometric detection of metal ions. Anal Chem. 2021;93(31):10834–40. https://doi.org/10.1021/acs.analchem.1c01077.
Liu MY, Lou XH, Du J, Guan M, Wang J, Ding XF, Zhao JL. DNAzyme-based fluorescent microarray for highly selective and sensitive detection of lead(II). Analyst. 2012;137(1):70–2. https://doi.org/10.1039/c1an15633k.
Wang L, Jin Y, Deng J, Chen GZ. Gold nanorods-based FRET assay for sensitive detection of Pb2+ using 8–17DNAzyme. Analyst. 2011;136(24):5169–74. https://doi.org/10.1039/c1an15783c.
Wang JL, Chen SH, Yuan R, Hu FX. DNA branched junctions induced the enhanced fluorescence recovery of FAM-labeled probes on rGO for detecting Pb2+. Anal Bioanal Chem. 2020;412(11):2455–63. https://doi.org/10.1007/s00216-020-02458-2.
Guo Y, Li JT, Zhang XQ, Tang YL. A sensitive biosensor with a DNAzyme for lead(II) detection based on fluorescence turn-on. Analyst. 2015;140:4642. https://doi.org/10.1039/c5an00677e.
Funding
This work was financially supported by National Natural Science Foundation of China (31571919), National Natural Science Foundation of China (31901777), Natural Science Foundation of Jilin Province (20200201218JC), and Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0927).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Liu, Z., Li, Y. et al. Signal-on fluorescent sensing strategy for Pb2+ detection based on 8–17 DNAzyme-mediated molecular beacon-type catalytic hairpin assembly circuit. Anal Bioanal Chem 414, 6581–6590 (2022). https://doi.org/10.1007/s00216-022-04218-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04218-w