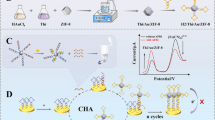
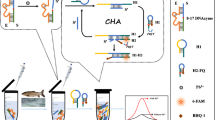
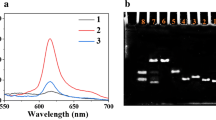
Abstract
Ochratoxin A (OTA) poses severe risks to the environment and human health, making the development of an accurate and sensitive analytical method for OTA detection essential. In this study, a catalytic hairpin assembly (CHA)–based Förster resonance energy transfer (FRET) aptasensor was developed to detect OTA using carbon quantum dots (CDs) and 6-carboxy-fluorescein (FAM) as dual signal readout. In the presence of OTA, the aptamer specifically interacted with OTA to release the helper DNA (HP), which could open the hairpin structure of FAM-labeled hairpin DNA 1 (H1-FAM) modified on the surface of gold nanoparticles (AuNPs). CHA between H1-FAM and hairpin H2 labeled with CDs (H2-CDs) can release HP for the next cycle, resulting in the occurrence of FRET with CDs as the energy donor and FAM as the energy acceptor. According to the ratio of FCDs/FFAM, the proposed aptasensor showed a wide linear range from 5.0 pg/mL to 3.0 ng/mL and a low detection limit of 1.5 pg/mL for OTA detection. Moreover, satisfactory results were obtained for OTA detection in rice, suggesting the potential application of this sensor in food safety analysis.





Similar content being viewed by others
References
Ha TH. Recent advances for the detection of ochratoxin A. Toxins. 2015;7:5276–300.
Zheng F, Ke W, Shi L, Liu H, Zhao Y. Plasmonic Au-Ag janus nanoparticle engineered ratiometric surface-enhanced raman scattering aptasensor for ochratoxin a detection. Anal Chem. 2019;91:11812–20.
Wu G, Xiong Z, Oh S-H, Ren Y, Wang Q, Yang L. Two-color, ultra-sensitive fluorescent strategy for ochratoxin A detection based on hybridization chain reaction and DNA tweezers. Food Chem. 2021;356:129663.
Liu L, Huang Q, Tanveer ZI, Jiang K, Zhang J, Pan H, Luan L, Liu X, Han Z, Wu Y. “Turn off-on” fluorescent sensor based on quantum dots and self-assembled porphyrin for rapid detection of ochratoxin A. Sens Actuators B Chem. 2020;302: 127212.
Khoshbin Z, Abnous K, Taghdisi SM, Verdian A, Sameiyan E, Ramezani M, Alibolandi M. An ultra-sensitive dual-responsive aptasensor with combination of liquid crystal and intercalating dye molecules: A food toxin case study. Food Chem. 2022;381:132265.
Hao L, Li M, Peng K, Ye T, Wu X, Yuan M, Cao H, Yin F, Gu H, Xu F. Fluorescence resonance energy transfer aptasensor of ochratoxin A constructed based on gold nanorods and DNA tetrahedrons. J Agric Food Chem. 2022;70:10662–8.
Li Z, Miao X, Xing K, Peng X, Zhu A, Ling L. Ultrasensitive electrochemical sensor for Hg2+ by using hybridization chain reaction coupled with Ag@Au core-shell nanoparticles. Biosens Bioelectron. 2016;80:339–43.
Zeng Z, Zhou R, Sun R, Zhang X, Cheng Z, Chen C, Zhu Q. Nonlinear hybridization chain reaction-based functional DNA nanostructure assembly for biosensing, bioimaging applications. Biosens Bioelectron. 2021;173:112814.
Lv W, Li C, Li Y, Zhen S, Huang C. Hierarchical hybridization chain reaction for amplified signal output and cascade DNA logic circuits. Anal Chem. 2021;93:3411–7.
Wang L, Zeng L, Wang Y, Chen T, Chen W, Chen G, Li C, Chen J. Electrochemical aptasensor based on multidirectional hybridization chain reaction for detection of tumorous exosomes. Sens Actuators B Chem. 2021;332:129471.
Miao X, Cheng Z, Ma H, Li Z, Xue N, Wang P. Label-free platform for microRNA detection based on the fluorescence quenching of positively charged gold nanoparticles to silver nanoclusters. Anal Chem. 2018;90:1098–103.
Zhang Y, Zhang X, Situ B, Wu Y, Luo S, Zheng L, Qiu Y. Rapid electrochemical biosensor for sensitive profiling of exosomal microRNA based on multifunctional DNA tetrahedron assisted catalytic hairpin assembly. Biosens Bioelectron. 2021;183:113205.
Bodulev OL, Zhao S, Sakharov IY. Improving the sensitivity of the miRNA assay coupled with the mismatched catalytic hairpin assembly reaction by optimization of hairpin annealing conditions. Anal Chem. 2021;93:6824–30.
Li L, Lv W, Xu Y, Li Y, Li C, Huang C. DNA logic nanodevices for the sequential imaging of cancer markers through localized catalytic hairpin assembly reaction. Anal Chem. 2022;94:4399–406.
Nie Y, Jiang J, Peng K, Chai Y, Yuan R. Two kinds of DNA enzyme-powered bidirectional one-dimensional DNA walking nanomachine for payload release and biosensing. Biosens Bioelectron. 2021;175:112848.
Miao P, Chai H, Tang Y. DNA hairpins and dumbbell-wheel transitions amplified walking nanomachine for ultrasensitive nucleic acid detection. ACS Nano. 2022;16:4726–33.
Liu J, Ye L, Zhang Y, Yang H, Zhou L, Luo E, Lei J. Nonenzymatic target-driven DNA nanomachine for monitoring malathion contamination in living cells and bioaccumulation in foods. Anal Chem. 2022;94:5667–73.
Wang S, Zhang F, Chen C, Cai C. Ultrasensitive graphene quantum dots-based catalytic hairpin assembly amplification resonance light scattering assay for p53 mutant DNA detection. Sens Actuators B Chem. 2019;291:42–7.
Bodulev OL, Burkin KM, Efremov EE, Sakharov IY. One-pot microplate-based chemiluminescent assay coupled with catalytic hairpin assembly amplification for DNA detection. Anal Bioanal Chem. 2020;412:5105–11.
Liu X, Zhou X, Xia X, Xiang H. Catalytic hairpin assembly-based double-end DNAzyme cascade-feedback amplification for sensitive fluorescence detection of HIV-1 DNA. Anal Chim Acta. 2020;1096:159–65.
Wang L, Liu P, Liu Z, Zhao K, Ye S, Liang G, Zhu J-J. Simple tripedal DNA walker prepared by target-triggered catalytic hairpin assembly for ultrasensitive electrochemiluminescence detection of microRNA. ACS Sens. 2020;5:3584–90.
Dai J, Xing C, Lin Y, Huang Y, Yang Y, Chen Z, Lu C, Yang H. Localized DNA catalytic hairpin assembly reaction on DNA origami for tumor-associated microRNA detection and imaging in live cells. Sens Actuators B Chem. 2020;344: 130195.
Chai H, Wang M, Tang L, Miao P. Ultrasensitive electrochemical detection of miRNA coupling tetrahedral DNA modified gold nanoparticles tags and catalyzed hairpin assembly. Anal Chim Acta. 2021;1165: 338543.
Huang Q, Ma P, Li H, Yin B, Ye B. Catalytic-hairpin-assembly-assisted DNA tetrahedron nanoprobe for intracellular microRNA imaging. ACS Appl Bio Mater. 2020;3:2861–6.
Li N, Du M, Liu Y, Ji X, He Z. Multipedal DNA walker biosensors based on catalyzed hairpin assembly and isothermal strand-displacement polymerase reaction for the chemiluminescent detection of proteins. ACS Sens. 2018;3:1283–90.
Chai H, Cheng W, Xu L, Gui H, He J, Miao P. Fabrication of polymeric ferrocene nanoparticles for electrochemical aptasensing of protein with target-catalyzed hairpin assembly. Anal Chem. 2022;91:9940–5.
Yang P, Guo X, Zhang J, Chen C, Gan Y, Xie W, Du Y, Wu Z. Picomolar thrombin detection by orchestration of triple signal amplification strategy with hierarchically porous Ti3C2Tx MXene electrode material-catalytic hairpin assembly reaction-metallic nanoprobes. Biosens Bioelectron. 2022;208: 114228.
Wang D, Guo R, Wei Y, Zhang Y, Zhao X, Xu Z. Sensitive multicolor visual detection of telomerase activity based on catalytic hairpin assembly and etching of Au nanorods. Biosens Bioelectron. 2018;122:247–53.
Lu H, Zhao W, Xu J, Chen H. Visual electrochemiluminescence ratiometry on bipolar electrode for bioanalysis. Biosens Bioelectron. 2018;102:624–30.
Huo X, Lu H, Xu J, Zhou H, Chen H. Recent advances of ratiometric electrochemiluminescence biosensors. J Mater Chem B. 2019;7:6469–75.
Wu Y, Huang S, Zeng F, Wang J, Yu C, Huang J, Xie H, Wu S. A ratiometric fluorescent system for carboxylesterase detection with AIE dots as FRET donors. Chem Commun. 2015;51:12791–4.
Chen L, Lee S, Lee M, Lim C, Choo J, Park JY, Lee S, Joo S-W, Lee K-H, Choi Y-W. DNA hybridization detection in a microfluidic channel using two fluorescently labelled nucleic acid probes. Biosens Bioelectron. 2008;23:1878–82.
Feng B, You J, Zhao F, Wei M, Liu Y, Yuan K, Suo Z. A ratiometric fluorescent aptamer homogeneous biosensor based on hairpin structure aptamer for AFB1 detection. J Fluoresc. 2022;32:1695–701.
Liu S, Bai J, Huo Y, Ning B, Peng Y, Li S, Han D, Kang W, Gao Z. A zirconium-porphyrin MOF-based ratiometric fluorescent biosensor for rapid and ultrasensitive detection of chloramphenicol. Biosens Bioelectron. 2020;149: 111801.
Sheng E, Lu Y, Tan Y, Xiao Y, Li Z, Dai Z. Ratiometric fluorescent quantum dot-based biosensor for chlorothalonil detection via an inner-filter effect. Anal Chem. 2020;92:4364–70.
Peng D, Li Y, Huang Z, Liang R, Qiu J, Liu J. Efficient DNA-catalyzed porphyrin metalation for fluorescent ratiometric Pb2+ detection. Anal Chem. 2019;91:11403–8.
Wang J, Li T, Shen R, Li G, Ling L. Polymerase chain reaction-dynamic light scattering sensor for DNA and protein by using both replication and cleavage properties of Taq polymerase. Anal Chem. 2019;91:3429–35.
Wang J, Li T, Li H, Li G, Wu S, Ling L. A universal colorimetric PCR biosensor based upon triplex formation with the aid of Ru(phen)2d ppx2+. Sens Actuators B Chem. 2019;278:39–45.
Funding
This work was supported by the National Natural Science Foundation of China (31671581) and the Natural Science Foundation of Henan Province (202300410206).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Wang, Y., Lin, Y. et al. A catalytic hairpin assembly–based Förster resonance energy transfer sensor for ratiometric detection of ochratoxin A in food samples. Anal Bioanal Chem 415, 867–874 (2023). https://doi.org/10.1007/s00216-022-04479-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04479-5