Abstract
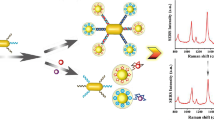
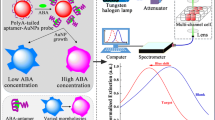
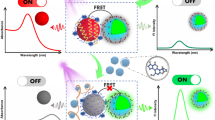
Abscisic acid (ABA), as the most common plant hormone in the growth of wheat, can greatly affect the yield when its levels deviate from normal. Therefore, highly sensitive and selective detection of this hormone is greatly needed. In this work, we developed an aptamer sensor based on surface-enhanced Raman spectroscopy (SERS) and applied it for the high sensitivity detection of ABA. Biotin-modified ABA aptamer complement chains were modified on ferrosoferric oxide magnetic nanoparticles (Fe3O4MNPs) and acted as capture probes, and sulfhydryl aptamer (SH-Apt)-modified silver-coated gold nanospheres (Au@Ag NPs) were used as signal probes. Through the recognition of the ABA aptamer and its complementary chains, an aptamer sensor based on SERS was constructed. As SERS internal standard molecules of 4-mercaptobenzoic acid (4-MBA) were encapsulated between the gold core and silver shell of the signal probes; the constructed aptamer sensor generated a strong SERS signal of 4-MBA after magnetic separation. When there were ABA molecules in the detection system, with the preferential binding of ABA aptamer and ABA molecule, the signal probes were released from the capture probes, after magnetic separation, leading to a linear decrease in SERS intensity of 4-MBA. Thus, the detection response was linear over a logarithmic concentration range, with an ultra-low detection limit of 0.67 fM. In addition, the practical use of this assay method was demonstrated in ABA detection from fresh wheat leaves, with a relative error (RE) of 5.43–8.94% when compared with results from enzyme-linked immunosorbent assay (ELISA). The low RE value proves that the aptamer sensor will be a promising method for ABA detection.
Graphical abstract








Similar content being viewed by others
References
Silveira V, Santa-Catarina C, Balbuena TS, Moraes FMS, Ricart CAO, Sousa MV, Guerra MP, Handro W, Floho EIS. Endogenous abscisic acid and protein contents during seed development of Araucaria angustifolia. Biol Plant. 2008;52(1):101–4.
Shibata M, Coelho CMM, de Garighan JA, dos Santos HP, Araldi CG, Maraschin M. Seed development of Araucaria angustifolia: plant hormones and germinability in 2 years of seeds production. New Forest. 2021;52(5):759–75.
Sirko A, Wawrzynska A, Brzywczy J, Sienko M. Control of ABA signaling and crosstalk with other hormones by the selective degradation of pathway components. Int J Mol Sci. 2021;22(9):4638.
De Y, Shi FL, Gao FQ, Mu HB, Yan WH. Siberian Wildrye (Elymus sibiricus L.) abscisic acid-insensitive 5 gene is involved in abscisic acid-dependent salt response. Plant Basel. 2021;10(7):1351.
Zhu YC, Wang QY, Gao ZW, Wang Y, Liu YJ, Ma ZP, Chen YW, Zhang YC, Yan F, Li JW. Analysis of Phytohormone signal transduction in Sophora alopecuroides under salt stress. Int J Mol Sci. 2021;22(14):7313.
McAdam SAM, Sussmilch FC. The evolving role of abscisic acid in cell function and plant development over geological time. Semin Cell Dev Biol. 2021;109:39–45 SI.
Yang JF, Chen MX, Zhang JH, Hao GF, Yang GF. Structural dynamics and determinants of abscisic acid-receptor binding preference in different aggregation states. J Exp Bot. 2021;72(13):5051–65.
Zhou GH, Luo YZ, Xu QF, Ji XH, He ZK. Peptide-capped gold nanoparticle for colorimetric immunoassay of conjugated abscisic acid. ACS Appl Mater Interfaces. 2012;4(9):5010–5.
Wang S, Zhang H, Zephania B, Li DX, Li SX, Wang L, Shang JJ, Hu JD. A multi-channel localized surface plasmon resonance system for absorptiometric determination of abscisic acid by using gold nanoparticles functionalized with a polyadenine-tailed aptamer. Microchim Acta. 2020;187(1):20.
Yan SJ, Guo LW, Wu CE, Liang LY, Wang JD, Zhang LN. Simultaneous determination of three kinds of endogenous hormones content in seeds of post-harvest Yali pear by high performance liquid chromatography. Chin J Anal Chem. 2010;38(6):843–7.
Qi BB, Wu C, Liang HL, Cui KH, Fahad S, Wang ML, Liu BY, Nie LX, Huang JL, Tang H. Optimized high-performance liquid chromatography method for determining nine Cytokinins, Indole-3-acetic acid and abscisic acid. Sustainability. 2021;13(13):6998.
Zhou GH, Wang P, Yuan J, Qiu T, He ZK. Immunomagnetic assay combined with CdSe/ZnS amplification of chemiluminescence for the detection of abscisic acid. Sci China Che. 2011;54(8):1298–303.
Fu JH, Sun XH, Wang JD, Chu JF, Yan CY. Progress in quantitative analysis of plant hormones. Chin Sci Bull. 2011;56(4–5):355–66.
Wang PX, Sun Y, Li X, Wang L, Xu Y, Li GL. Recent advances in metal organic frameworks based surface enhanced Raman scattering substrates: synthesis and applications. Mol. 2021;26(1):209.
Fleischmann MP, Hendra PJ. Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys. 1974;26:163–6.
Chen YF, Yang J, Li ZL, Li R, Ruan WD, Zhuang ZP, Zhao B. Experimental and density functional theory study of Raman and SERS spectra of 5-amino-2-mercaptobenzimidazole. Spectrochimi Acta Part A. 2016;153:344–8.
Badillo-Ramirez I, Saniger JM, Popp J, Cialla-May D. SERS characterization of dopamine and in situ dopamine polymerization on silver nanoparticles. Phys Chem Chem Phys. 2021;23(21):12158–70.
Makam P, Shilpa R, Kandjani AE, Periasamy SR, Sabri YM, Madhu C, Bhargava SK, Govindaraju T. SERS and fluorescence-based ultrasensitive detection of mercury in water. Biosens Bioelectron. 2018;100:556–64.
Sheng EZ, Lu YX, Xiao Y, Li ZX, Wang HS, Dai ZH. Simultaneous and ultrasensitive detection of three pesticides using a surface-enhanced Raman scattering-based lateral flow assay test strip. Biosens Bioelectron. 2021;181:113149.
Fan MK, Andrade GFS, Brolo AG. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal Chim Acta. 2020;1097:1–29.
Li JF, Zhang YJ, Ding SY, Rajapandiyan P, Tian ZQ. Core−Shell nanoparticle-enhanced Raman spectroscopy. Che Rev. 2017;117(7):5002–69.
Kim J, Sim K, Cha S, Oh JW, Nam JM. Single-particle analysis on Plasmonic Nanogap Systems for Quantitative SERS. J Raman Spectrosc. 2020;52(2):375–85.
Wang XA, Shen W, Zhou BB, Yu DY, Tang XH, Liu JH, Huang XJ. The rationality of using core-shell nanoparticles with embedded internal standards for SERS quantitative analysis based glycerol-assisted 3D hotspots platform. RSC Adv. 2021;11(33):20326–34.
Chen RP, Du X, Cui YJ, Zhang XY, Ge QY, Dong J, Zhao XW. Vertical flow assay for inflammatory biomarkers based on Nanofluidic Channel Array and SERS Nanotags. Small. 2020;16(32):2002801.
Dai X, Song ZL, Song WJ, Zhang JL, Fan GC, Wang W, Luo XL. Shell-switchable SERS blocking strategy for reliable signal-on SERS sensing in living cells: detecting an external target without affecting the internal Raman molecule. Anal Chem. 2020;92(16):11496–75.
Lin S, Lin X, Han S, Liu YL, Hasi W, Wang L. Flexible fabrication of a paper-fluidic SERS sensor coated with a monolayer of core-shell nanospheres for reliable quantitative SERS measurements. Anal Chim Acta. 2020;1108:167–76.
Song MY, Khan IM, Wang ZP. Research Progress of optical Aptasensors based on AuNPs in food safety. Food Anal Methods. 2021;14(10):12161.
Ma XY, Lin XC, Xu XM, Wang ZP. Fabrication of gold/silver nanodimer SERS probes for the simultaneous detection of salmonella typhimurium and Staphylococcus aureus. Mikrochim Acta. 2021;188(6):202.
Zhou ZH, Xiao R, Cheng SY, Wang S, Shi LL, Wang CW, Qi KZ, Wang SQ. A universal SERS-label immunoassay for pathogen bacteria detection based on Fe3O4@au-aptamer separation and antibody-protein a orientation recognition. Anal Chim Acta. 2021;1160:338421.
Muhammad M, Huang Q. A review of aptamer-based SERS biosensors: design strategies and applications. Talanta. 2021;277:122188.
Man TT, Lai W, Xiao MS, Wang XW, Chandrasekaran AR, Pei H, Li L. A versatile biomolecular detection platform based on photo-induced enhanced Raman spectroscopy. Biosens Bioelectron. 2019;147:111742.
Grozio A, Gonzalez VM, Millo E, Sturla L, Vigliarolo T, Bagnasco L, Guida G, D'Arrigo C, Flora AD, Salis A, Martin EM, Bellotti M, Zocchi E. Selection and characterization of single stranded DNA aptamers for the hormone abscisic acid. Nucleic Acid Thera. 2013;23(5):322–31.
Zhang CY, Huang LJ, Pu HB, Sun DW. Magnetic surface-enhanced Raman scattering (MagSERS) biosensors for microbial food safety: fundamentals and applications. Trends in Food Sci Tech. 2021;113:366–81.
Yang EL, Li D, Yin PK, Xie QY, Li Y, Lin QY, Duan YX. A novel surface-enhanced Raman scattering (SERS) strategy for ultrasensitive detection of bacteria based on three-dimensional (3D) DNA walker. Biosens Bioelectron. 2020;172:112758.
Ning CF, Tian YF, Zhou W, Yin BC, Ye BC. Ultrasensitive SERS detection of specific oligonucleotides based on au@AgAg bimetallic nanorods. Analyst. 2019;144(9):2929–35.
Wang KQ, Sun DW, Pu HB, Wei QY. Shell thickness-dependent au@ag nanoparticles aggregates for high-performance SERS applications. Talanta. 2019;195:506–5.
Jiang CH, Wang Y, Song W, Lu LH. Delineating the tumor margin with intraoperative surface-enhanced Raman spectroscopy. Anal Bioanal Chem. 2019;411(18):3993–4006.
Wang S, Li W, Chang KK, Liu J, Guo QQ, Sun HF, Jiang M, Zhang H, Chen J, Hu JD. Localized surface plasmon resonance-based abscisic acid biosensor using aptamer-functionalized gold nanoparticles. PLoS One. 2017;12(9):e0185530.
He DY, Wu ZZ, Cui B, Jin ZY. A novel SERS-based aptasensor for ultrasensitive sensing of microcystin-LR. Food Chem. 2018;278:197–202.
He HR, Da W, Pu HB, Huang LJ. Bridging Fe3O4@au nanoflowers and au@ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B1. Food Chem. 2020;324:126832.
Liu X, Ma L, Lin YW, Lu YT. Determination of abscisic acid by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr A. 2003;1021(1–2):209–13.
Funding
The authors are thankful to the National Natural Science Foundation of China (grant numbers 32071890, 31671581) and Agricultural Biological resources Engineering technology Foreign Scientist Workshop project of Henan province (GZS2021007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 1244 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, L., Zhang, H. et al. Ultrasensitive detection of plant hormone abscisic acid-based surface-enhanced Raman spectroscopy aptamer sensor. Anal Bioanal Chem 414, 2757–2766 (2022). https://doi.org/10.1007/s00216-022-03923-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-03923-w