Abstract
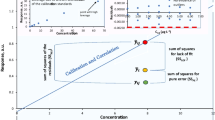
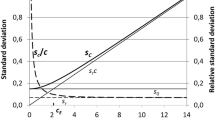
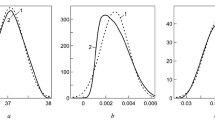
Analytical chemists in the food sector are well acquainted with the empirically observed Horwitz function. It relates reproducibility (interlaboratory) standard deviations estimated by collaborative trial with the mass fraction of the analyte. No compelling theory accounts for the power-law form of the function or its parameters. The equation of Zitter and God, ostensibly covering the same ground, is less well known but, in spite of its secure foundation in theory and practice, seems at first sight to be incompatible with the Horwitz function. In this paper, we see that because of the limited range of mass fractions in which a single collaborative trial is usually conducted, collections of statistics from collaborative trials exhibit the Horwitz function over the mass fraction range from about 10−7 (100 ppb) to about 10−1 (10%).
Graphical abstract





Similar content being viewed by others
References
Horwitz W, Kamps LR, Boyer KW. Quality assurance in the analysis of foods and trace constituents. J Assoc Off Anal Chem. 1980;63:1344–654.
Thompson M. Recent trends in inter-laboratory precision at ppb and sub-ppb concentrations in relation to fitness for purpose criteria in proficiency testing. Analyst. 2000;125:385–6.
Thompson M. An emergent optimal precision in chemical measurement at low concentrations. Anal Meth. 2013;5:4518–9.
Albert R, Horwitz W. A heuristic derivation of the Horwitz curve. Anal Chem. 1997;69:789–90.
Thompson M. A natural history of analytical methods. Analyst. 1999;124:991.
Zitter H, God C. Ermittlung, Auswertung und Ursachen von Fehlern bei Betriebsanalysen. Fresenius Z Anal Chem. 1971;255:1–9.
Thompson M. Uncertainty functions, a compact way of summarising or specifying the behaviour of analytical systems. TrAC Trends Anal Chem. 2011;30:1168–75.
Horwitz W. Protocol for the design, conduct and interpretation of method-performance studies Pure Appl. Chem. 1995;67:331–43.
Thompson M, Potts PJ, Webb PC, Kane JS. GeoPT - a proficiency test for geoanalysis. Analyst. 1997;122:1249–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thompson, M. Towards an explanation of the Horwitz function. Anal Bioanal Chem 414, 1671–1676 (2022). https://doi.org/10.1007/s00216-021-03791-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03791-w