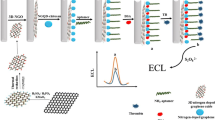
Abstract
In this study, reduced graphene oxide (rGO) hybridized high internal phase emulsions were developed and polymerized as porous carriers for aptamer (5’/5AmMC6/-AGT CCG TGG TAG GGC AGG TTG GGG TGA CT-3’) modification to enrich human α-thrombin from serum. The structure and properties of the materials were confirmed by scanning electron microscope (SEM), Fourier transform infrared spectroscope (FT-IR), and X-ray photoelectron spectra (XPS). The adsorption ability and selectivity were studied and the thrombin was detected with liquid chromatography-mass spectrometry (LC–MS). The adsorption of thrombin onto the sorbent was achieved within 30 min and the desorption was realized using 5.0 mL of acetonitrile/water (80/20, v/v). The thrombin was quantified by LC–MS according to its characteristic peptide sequence of ELLESYIDGR.
Graphical abstract






Similar content being viewed by others
References
Hashemi B, Zohrabi P, Shamsipur M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta. 2018;187:337–47.
Lissant KJ, Mayhan KG. A study of medium and high internal phase ratio water/polymer emulsions. J Colloid Interface Sci. 1973;42:201–8.
Zhang W, Ruan G, Li X, Jiang X, Huang Y, Du F, Li J. Novel porous carbon composites derived from a graphene-modified high-internal-phase emulsion for highly efficient separation and enrichment of triazine herbicides. Anal Chim Acta. 2019;1017:17–24.
Silverstein MS. PolyHIPEs: recent advances in emulsion-templated porous polymers. Prog Polym Sci. 2014;39:199–234.
Du F, Sun L, Zhen X, Nie H, Zheng Y, Ruan G, Li J. High-internal-phase-emulsion polymeric monolith coupled with liquid chromatography-electrospray tandem mass spectrometry for enrichment and sensitive detection of trace cytokinins in plant samples. Anal Bioanal Chem. 2015;407:6071–9.
Jiang X, Gui Ruan, Deng H, Gan Z, Zhang W, Du F, Chen Z. Synthesis of amphiphilic and porous copolymers through polymerization of high internal phase carboxylic carbon nanotubes emulsions and application as adsorbents for triazine herbicides analysis. Chem Eur J. 2021;415:129005.
López-Diaz D, Merchán MD, Velázquez MM. The behavior of graphene oxide trapped at the air water interface. Adv Colloid Interface Sci. 2020;286:102312.
Verdian A, Fooladi E, Rouhbakhsh Z. Recent progress in the development of recognition bioelements for polychlorinated biphenyls detection: antibodies and aptamers. Talanta. 2019;202:123–35.
Du F, Ruan G, Liang S, Xie F, Liu H. Monolithic molecularly imprinted solid-phase extraction for the selective determination of trace cytokinins in plant samples with liquid chromatography-electrospray tandem mass spectrometry. Anal Bioanal Chem. 2012;404:489–501.
Yu Q, Liu S, Zheng F, Hua Xiao, Guan H, Feng Y. Identification and quantification of benzimidazole metabolites of thiophonate-methyl sprayed on celery cabbage using SiO2@NiO solid-phase extraction in combination with HPLC-MS/MS. Chin Chem Lett. 2020;31:482–6.
Jiang D, Hu T, Zheng H, Xu G, Jia Q. Aptamer-functionalized magnetic conjugated organic frameworks for selective extraction of trace hydroxylated polychlorinated biphenyls in human serum. Chem Eur J. 2018;24:10390–6.
Climent E, Rurack K. Combining electrochemiluminescence detection with aptamer-gated indicator releasing mesoporous nanoparticles enables ppt sensitivity for strip-based rapid tests. Angew Chem Int Ed. 2021;60:2–13.
Liu L, Yang K, Gao H, Li X, Chen Y, Zhang L, Peng X, Zhang Y. Artificial antibody with site-enhanced multivalent aptamers for specific capture of circulating tumor cells. Anal Chem. 2019;91:2591–4.
Du F, Alam MN, Pawliszyn J. Aptamer-functionalized solid phase microextraction-liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal Chim Acta. 2014;845:45–52.
Deng N, Liang Z, Liang Y, Sui Z, Zhang L, Wu Q, Yang K, Zhang L, Zhang Y. Aptamer modified organic-inorganic hybrid silica monolithic capillary columns for highly selective recognition of thrombin. Anal Chem. 2012;84:10186–90.
Xue J, Zhao Q, Yang L, Ma H, Wu D, Liu L, Ren X, Ju H, Wei Q. Dual-mode sensing platform guided by intramolecular electrochemiluminescence of a ruthenium complex and cationic N, N-Bis(2-(trimethylammonium iodide)propylene) perylene-3,4,9,10-tetracarboxydiimide for estradiol assay. Anal Chem. 2021;93:6088–93.
Chang M, Wang Q, Qin W, Shi X, Xu G. Rational synthesis of aptamer-functionalized polyethylenimine modified magnetic graphene oxide composites for highly efficient enrichment and comprehensive metabolomics analysis of exosomes. Anal Chem. 2020;92:15497–505.
Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64.
Jiang N, Zhu T, Hu Y. Competitive aptasensor with gold nanoparticle dimers and magnetite nanoparticles for SERS-based determination of thrombin. Microchim Acta. 2019;186:747.
Xing Y, Han J, Wu X, Pierce DT, Zhao JX. Graphene/gold nanoparticle composites for ultrasensitive and versatile biomarker assay using single-particle inductively-coupled plasma/mass spectrometry. Analyst. 2020;145:7932–40.
Cui H, Fu X, Yang L, Xing S, Wang XF. 2D titanium carbide nanosheets based fluorescent aptasensor for sensitive detection of thrombin. Talanta. 2021;228:122219.
Chen Z, Sun M, Luo F, Xu K, Lin Z, Zhang L. Stimulus-response click chemistry based aptamer-functionalized mesoporous silica nanoparticles for fluorescence detection of thrombin. Talanta. 2018;178:563–8.
Kaneko K, Hara M, Nishino T, Maruyama T. One-step biotinylation of cellulose paper by polymer coating to prepare a paper-based analytical device. Anal Chem. 2020;92:1978–87.
Ge H, Bao H, Zhang L, Chen G. Preparation of porous graphene using cuprous oxide microspheres as sacrificial templates for enriching proteins and peptides. Carbon. 2015;82:579–89.
Huang Y, Ruan G, Ruan Y, Zhang W, Li X, Du F, Hu C, Li J. Hypercrosslinked porous polymers hybridized with graphene oxide for water treatment: dye adsorption and degradation. RSC Adv. 2018;8:13417–22.
Kimmins SD, Cameron NR. Functional porous polymers by emulsion templating: recent advances. Adv Funct Mater. 2011;21:211–25.
Yang G, Chen H, Qin H, Feng Y. Amination of activated carbon for enhancing phenol adsorption: effect of nitrogen-containing functional groups. Appl Surf Sci. 2014;293:299–305.
Inagaki M, Toyoda M, Soneda Y, Morishita T. Nitrogen-doped carbon materials. Carbon. 2018;132:104–40.
Villalonga A, Pérez-Calabuig AM, Villalonga R. Electrochemical biosensors based on nucleic acid aptamers. Anal Bioanal Chem. 2020;412:55–72.
Dolot R, Lam CH, Sierant M, Zhao Q, Liu FW, Nawrot B, Egli M, Yang X. Crystal structures of thrombin in complex with chemically modified thrombin DNA aptamers reveal the origins of enhanced affinity. Nucleic Acids Res. 2018;46:4819–30.
Sandner A, Ngo K, Schiebel J, Pizarroso AIM, Schmidt L, Wenzel B, Steinmetzer T, Ostermann A, Heine A, Klebe G. How a fragment draws attention to selectivity discriminating features between the related proteases trypsin and thrombin. J Med Chem. 2021;64:1611–25.
Lai PX, Mao JY, Unnikrishnan B, Chu HW, Wu CW, Chang HT, Huang CC. Self-assembled, bivalent aptamers on graphene oxide as an efficient anticoagulant. Biomater Sci. 2018;6:1882–91.
Xiong Y, Deng C, Zhang X. Development of aptamer-conjugated magnetic graphene/gold nanoparticle hybrid nanocomposites for specific enrichment and rapid analysis of thrombin by MALDI-TOF MS. Talanta. 2014;129:282–9.
Li D, Song Q, Li T, Shu C, Ji S, Su C, Su Y, Ding L. An LC-MS/MS method for protein detection based on a mass barcode and dual-target recognition strategy. RSC Adv. 2020;10:16094–100.
Guo WJ, Yang XY, Wu Z, Zhang ZL. A colorimetric and electrochemical dual-mode biosensor for thrombin using a magnetic separation technique. J Mater Chem B. 2020;8:3574–81.
Qin B, Yang K. Voltammetric aptasensor for thrombin by using a gold microelectrode modified with graphene oxide decorated with silver nanoparticles. Microchim Acta. 2018;185:407.
Zhao Q, Li XF, Le XC. Aptamer capturing of enzymes on magnetic beads to enhance assay specificity and sensitivity. Anal Chem. 2011;83:9234–6.
Funding
This research was supported by the National Natural Science Foundation of China (No. 21665006); the Natural Science Foundation from Guangxi Zhuang Autonomous Region (No. 2020GXNSFDA297025), the project of improving the basic scientific research ability of young and middle-aged teachers in Guangxi Universities (No. 2020KY12004), and Guangxi science and technology base and talent special project (No. 2019AC20330), respectively.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, W., Hu, H., Ruan, G. et al. Aptamer functionalized and reduced graphene oxide hybridized porous polymers SPE coupled with LC–MS for adsorption and detection of human α-thrombin. Anal Bioanal Chem 414, 1553–1561 (2022). https://doi.org/10.1007/s00216-021-03776-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03776-9