Abstract
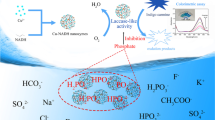
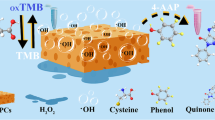
The rapid development of nanozymes for ultrasensitive detection of contaminate has resulted in considerable attention. Herein, a carboxyl- and aminopropyl-functionalized copper organophyllosilicate (Cu-CAP) was synthesized by a facile, one-pot sol–gel method. The bifunctional groups endow it with superior catalytic activity than that of natural enzyme. Besides, it possesses outstanding catalytic stability under harsh conditions such as high temperature, extremely high or low pH, and high salinity. Apart from laccase-mimetic activity, Cu-CAP also shows oxidation of the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) to the blue-colored TMBox in the presence of H2O2, which is similar to natural horseradish peroxidase (HRP). Interestingly, this colorimetric system was suppressed by hydroquinone (HQ) specifically. Inspired by this, Cu-CAP was used to develop a highly sensitive and selective colorimetric method for the determination of HQ. This assay displayed an extremely low detection limit of 23 nM and was applied for the detection of HQ in environmental water with high accuracy. This approach offers a new route for the rational design of high performance nanozymes for environmental and biosensing applications.
Graphical abstract







Similar content being viewed by others
References
Promsuwan K, Kaewjunlakan C, Saichanapan J, Soleh A, Limbut W. Poly(phenol red) hierarchical micro-structure interface enhanced electrode kinetics for adsorption and determination of hydroquinone. Electrochim Acta. 2021;377: 138072. https://doi.org/10.1016/j.electacta.2021.138072.
Wang, Yu-Min, Jiang, Jian-Hui, Zhang, Chong-Hua, et al. Conjugated polymer nanoparticles-based fluorescent biosensor for ultrasensitive detection of hydroquinone. Anal Chim Acta. 2018:60–5.https://doi.org/10.1016/j.aca.2018.01.027
Wittig J, Wittemer S, Veit M. Validated method for the determination of hydroquinone in human urine by high-performance liquid chromatography-coulometric-array detection. J Chromatogr B. 2001;761(1):125–32. https://doi.org/10.1016/S0378-4347(01)00321-8.
Sakodinskaya IK, esiderio CD, Nardi A, Fanali S. Micellar electrokinetic chromatographic study of hydroquinone and some of its ethers. Determination of hydroquinone in skin-toning cream. J Chromatogr. 1992;596(1):95–100. https://doi.org/10.1016/0021-9673(92)80208-C
Zhao XL, HY. Yao, XX. Xu, C. Liu, QY. Liu, ZX. Zhang, XX. Zhang, X. . Hydroquinone colorimetric sensing based on platinum deposited on CdS nanorods as peroxidase mimics. Microchimica Acta. 2020. https://doi.org/10.1007/s00604-020-04451-z
Sirajuddin, Bhanger MI, Niaz A, Shah A, Rauf A. Ultra-trace level determination of hydroquinone in waste photographic solutions by UV–vis spectrophotometry. Talanta. 2007;72(2):546–53. https://doi.org/10.1016/j.talanta.2006.11.021
Zenovia Moldovan DEP, Iulia Gabriela David, Mihaela Buleandra, Irinel Adriana Badea. A derivative spectrometric method for hydroquinone determination in the presence of kojic acid, glycolic acid, and ascorbic acid. J Spectro. 2017;2017:1–9. https://doi.org/10.1155/2017/6929520
Uddin S, Rauf A, Kazi TG, Afridi HI, Lutfullah G. Highly sensitive spectrometric method for determination of hydroquinone in skin lightening creams: application in cosmetics. Int J Cosmetic Sci. 2011;33(2):132–7. https://doi.org/10.1111/j.1468-2494.2010.00599.x.
García P, Santoro M, Kedor-Hackman E, Singh AK. Development and validation of a HPLC and a UV derivative spectrophotometric methods for determination of hydroquinone in gel and cream preparations. J Pharm Biomed Anal. 2005;39(3–4):764. https://doi.org/10.1016/j.jpba.2005.04.016.
Wang X, Zhao M, Song Y, Liu Q, Chen S. Synthesis of ZnFe2O4/ZnO heterostructures decorated three-dimensional graphene foam as peroxidase mimetics for colorimetric assay of hydroquinone. Sensor Actuat B-Chem. 2018;283:130–7. https://doi.org/10.1016/j.snb.2018.11.079.
Song, Yawen, Zhao, Minggang, Li, Hui, et al. Facile preparation of urchin-like NiCo2O4 microspheres as oxidase mimetic for colormetric assay of hydroquinone. Sensor Actuat B-Chem. 2018;255:1927–36. https://doi.org/10.1016/j.snb.2017.08.204
Ma Z, Li P, Lu Q, Liu M, Li H, Zhang Y, et al. Bifunctional colorimetric biosensors via regulation of the dual nanoenzyme activity of carbonized FeCo-ZIF. Sensor Actuat B-Chem. 2019;290:357–63. https://doi.org/10.1016/j.snb.2019.03.130.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotech. 2007;2(9):577–83. https://doi.org/10.1038/nnano.2007.260.
Sharifi M, Faryabi K, Talaei AJ, Shekha MS, Falahati M. Antioxidant properties of gold nanozyme: a review. J Mol Liq. 2020;297: 112004. https://doi.org/10.1016/j.molliq.2019.112004.
He W, Ying L, Yuan J, Yin JJ, Wu X, Hu X, et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials. 2011;32(4):1139–47. https://doi.org/10.1016/j.biomaterials.2010.09.040.
He L, Lu Y, Gao X, Song P, Huang Z, Liu S, et al. Self-cascade system based on cupric oxide nanoparticles as dual-functional enzyme mimics for ultrasensitive detection of silver ions. ACS Sustain Chem Eng. 2018;6(9):12132–9. https://doi.org/10.1021/acssuschemeng.8b02476.
Wang Y, Chen C, Zhang D, Wang J. Bifunctionalized Novel Co-V MMO Nanowires: intrinsic oxidase and peroxidase like catalytic activities for antibacterial application. Appl Catal B- Environ. 2019;261: 118256. https://doi.org/10.1016/j.apcatb.2019.118256.
Zhang P, Sun D, Cho A, Weon S, Choi W. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nature Comm. 2019;10(1):940. https://doi.org/10.1038/s41467-019-08731-y.
Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22(19):2206–10. https://doi.org/10.1002/adma.200903783.
Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. ChSRv. 2019;48:3683–704. https://doi.org/10.1039/C8CS00718G.
Liu Y, Wang X, Wei H. Light-responsive nanozymes for biosensing. Analyst. 2020;145:1–19. https://doi.org/10.1039/D0AN00389A.
Meng Y, Li W, Pan X, Gadd GM. Applications of nanozymes in the environment. Environ Sci Nano. 2020;7:1–41. https://doi.org/10.1039/C9EN01089K.
Khan S, Sharifi M, Bloukh SH, Edis Z, Falahati M. In vivo guiding inorganic nanozymes for biosensing and therapeutic potential in cancer, inflammation and microbial infections. Talanta. 2021;224: 121805. https://doi.org/10.1016/j.talanta.2020.121805.
Gomaa EZ. Nanozymes: a promising horizon for medical and environmental applications. JCS. 2021:1–23. https://doi.org/10.1007/s10876-021-02079-4
Xin LA, Lw A, Dan DB, Liang NA, Jp A, Xn A. Emerging applications of nanozymes in environmental analysis: opportunities and trends. TrAC, Trends Anal Chem. 2019;120:115653–65. https://doi.org/10.1016/j.trac.2019.115653.
Liu S, Li K, Shao D, Shen Q, Zheng X. Dual enzyme-like activities of transition metal-doped MnO2 nanocoatings and their dependence on the electronic band structure and ionic dissolution. ApSS. 2020;534(3): 147649. https://doi.org/10.1016/j.apsusc.2020.147649.
Lu M, Li B, Guan L, Li K, Lin Y. Carbon-shielded three-dimensional Co–Mn nanowire array anchored on Ni foam with dual-enzyme mimic performance for selective detection of ascorbic acid. ACS Sustain Chem Eng. 2019;7(18):15471–8. https://doi.org/10.1021/acssuschemeng.9b03095.
Liu X, Yang J, Cheng J, Xu Y, Chen W, Li Y. Facile preparation of four-in-one nanozyme catalytic platform and the application in selective detection of catechol and hydroquinone. Sensor Actuat B-Chem. 2021;337: 129763. https://doi.org/10.1016/j.snb.2021.129763.
Zha J, Zhang P, Qin Y, Chen T, Ye] F. Fabrication of CeVO4 as nanozyme for facile colorimetric discrimination of hydroquinone from resorcinol and catechol. Sensor Actuat B-Chem. 2017;247:469–78. https://doi.org/10.1016/j.snb.2017.03.042
An Y, Chen M, Xue Q, Liu W. Preparation and self-assembly of carboxylic acid-functionalized silica. JCIS. 2007;311(2):507–13. https://doi.org/10.1016/j.jcis.2007.02.084.
Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60(6):551–65. https://doi.org/10.1016/S0031-9422(02)00171-1.
Li Wang RP, Xue Liu, Chendi Heng, Yanni Miao, Wei Wang, Andrew Carrier, Ken Oakes and Xu Zhang. Nitrite-enhanced copper-based Fenton reactions for biofilm removal. Chem. Comm. 2021;57:5514–7. https://doi.org/10.1039/D1CC00374G
Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149(1):43–50. https://doi.org/10.1016/S0300-483X(00)00231-6.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, grant numbers 42061134018, 42011530085, and 41877323), the Russian Science Foundation (RSF, grant number 21–47-00019), the Sichuan Science and Technology Program (grant number 2019JDJQ0056), and the Postgraduate Innovation Fund Project by Southwest University of Science and Technology (grant number 20YCX0022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, R., Sun, S., Liu, J. et al. Bifunctional nanozyme of copper organophyllosilicate for the ultrasensitive detection of hydroquinone. Anal Bioanal Chem 414, 1039–1048 (2022). https://doi.org/10.1007/s00216-021-03728-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03728-3