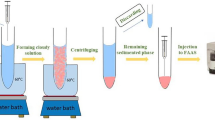
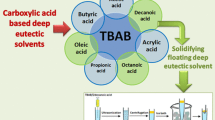
Abstract
Current trends in analytical chemistry encourage the use of innocuous solvents to develop modern methods aligned with green chemistry. In this sense, natural deep eutectic solvents (NADESs) have emerged as a novel generation of green solvents which can be employed in sample treatments as an alternative to the toxic organic solvents commonly used so far. In this work, a new extraction method employs dispersive liquid-liquid microextraction based on a solid floating organic droplet (DLLME-SFO), by using a mixture composed of a less dense than water extraction solvent, 1-dodecanol, and a novel dispersive solvent, NADES. The methodology was proposed to extract and preconcentrate some pesticide residues (fipronil, fipronil-sulfide, fipronil-sulfone, and boscalid) from environmental water and white wine samples before analysis by liquid-chromatography coupled to ultraviolet detection (HPLC-UV). Limits of quantification (LOQs) lower than 4.5 μg L−1, recoveries above 80%, and precision, expressed as RSD, below 15% were achieved in both samples showing that the proposed method is a powerful, efficient, and green alternative for the determination of these compounds and, therefore, demonstrating a new application for NADES in sample preparation. In addition, the DLLME-SFOD-HPLC-UV method was evaluated and compared with other reported approaches using the Analytical GREEnness metric approach, which highlighted the greenness of the proposed method.
Graphical abstract





Similar content being viewed by others
References
Cvjetko Bubalo M, Vidović S, Radojčić Redovniković I, Jokić S. Green solvents for green technologies. J Chem Technol Biotechnol. 2015. https://doi.org/10.1002/jctb.4668.
Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte AR. Natural deep eutectic solvents – solvents for the 21st century. ACS Sustain Chem. 2014. https://doi.org/10.1021/sc500096j.
Espino M, Fernández MA, Gómez FJV, Silva MF. Natural designer solvents for greening analytical chemistry. TrAC-Trends Anal Chem. 2015. https://doi.org/10.1016/j.trac.2015.11.006.
Gómez FJV, Espino M, Fernández MA, Boiteux J, Silva MF. DES-mediated approaches toward green analytical chemistry. In: Ramón DJ, Guillena G, editors. Deep eutectic solvents: synthesis, properties, and applications. 2020; pp.321–334.
Oldroyd BP. What’s killing American honey bees? PLoS Biol. 2007. https://doi.org/10.1371/journal.pbio.0050168.
Jacques A, Laurent M, EPILOBEE Consortium, Ribière-Chabert M, Saussac M, Bougeard S, et al. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0172591.
Requier F, Antúnez K, Morales CL, Aldea Sánchez P, Castilhos D, Garrido PM, Giacobino A, Reynaldi FJ, Rosso Londoño JM, Santos E, Garibaldi LA. Trends in beekeeping and honey bee colony losses in Latin America. J Apic Res. 2018. https://doi.org/10.1080/00218839.2018.1494919.
Bee Alert Geolocator. http://www.semabelhasemalimento.com.br/beealert/. (Accessed, 10 april 2021).
Castilhos D, Dombroski JLD, Bergamo GC, Gramacho KP, Gonçalves LS. Neonicotinoids and fipronil concentrations in honeybees associated with pesticide use in Brazilian agricultural areas. Apidologie. 2019. https://doi.org/10.1007/s13592-019-00676-x.
Informe sobre la mortandad de abejas en Córdoba. Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA). https://www.argentina.gob.ar/senasa/informe-sobre-la-mortandad-de-abejas-en-cordoba. (Accessed, 10 April 2021).
Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerdema RS. Environmental fate and toxicology of fipronil. J Pestic Sci. 2007. https://doi.org/10.1584/jpestics.R07-02.
Tingle CCD, Rother JA, Dewhurst CF, Lauer S, King WJ. Fipronil: environmental fate, ecotoxicology, and human health concerns. In: Ware GW, editor. Reviews of environmental contamination and toxicology. New York: Springer; 2003. p. 1–66.
Taylor MA. Recent developments in ectoparasiticides. Vet J. 2001. https://doi.org/10.1053/tvjl.2000.0549.
Holder PJ, Jones A, Tyler CR, Cresswell JE. Fipronil pesticide as a suspect in historical mass mortalities of honey bees. Proc Natl Acad Sci. 2018. https://doi.org/10.1073/pnas.1804934115.
Zaluski R, Kadri SM, Alonso DP, Martins Ribolla PE, de Oliveira Orsi R. Fipronil promotes motor and behavioral changes in honey bees (Apis mellifera) and affects the development of colonies exposed to sublethal doses. Environ Toxicol Chem. 2015. https://doi.org/10.1002/etc.2889.
Charreton M, Decourtye A, Henry M, Rodet G, Sandoz JC, Charnet P, Collet C. A locomotor deficit induced by sublethal doses of pyrethroid and neonicotinoid insecticides in the honeybee Apis mellifera. PLoS One. 2015. https://doi.org/10.1371/journal.pone.0144879.
Zhao X, Yeh JZ, Salgado VL, Narahashi T. Sulfone metabolite of fipronil blocks γ-aminobutyrica acid- and glutamate-activated chloride channels in mammalian and insect neurons. J Pharmacol Exp Ther. 2005. https://doi.org/10.1124/jpet.104.077891.
Roques BB, Lacroix MZ, Puel S, Gayrard V, Picard-Hagen N, Jouanin I, Perdu E, Martin PG, Viguié C. CYP450-dependent biotransformation of the insecticide fipronil into fipronil sulfone can mediate fipronil-induced thyroid disruption in rats. Toxicol Sci. 2012. https://doi.org/10.1093/toxsci/kfs094.
Avenot HF, Michailides TJ. Resistance to boscalid fungicide in Alternaria Alternata isolates from pistachio in California. Plant Dis. 2007. https://doi.org/10.1094/PDIS-91-10-1345.
Degrandi-Hoffman G, Chen Y, Watkins Dejong E, Chambers ML, Hidalgo G. Effects of oral exposure to fungicides on honey bee nutrition and virus levels. J Econ Entomol. 2015. https://doi.org/10.1093/jee/tov251.
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, van Engelsdorp D, Pettis JS. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One. 2010. https://doi.org/10.1371/journal.pone.0009754.
Simon-Delso N, San Martin G, Bruneau E, Hautier L. Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-24746-9.
Tsvetkov N, Samson-Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V, Zayed A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science. 2017. https://doi.org/10.1126/science.aam7470.
Das PC, Cao Y, Cherrington N, Hodgson E, Rose RL. Fipronil induces CYP isoforms and cytotoxicity in human hepatocytes. Chem Biol Interact. 2006. https://doi.org/10.1016/j.cbi.2006.09.013.
Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Gaulson D, Kreutzweiser DP, et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res. 2015. https://doi.org/10.1007/s11356-014-3471-x.
Hayasaka D, Korenaga T, Suzuki K, Saito F, Sánchez-Bayo F, Goka K. Cumulative ecological impacts of two successive annual treatments of imidacloprid and fipronil on aquatic communities of paddy mesocosms. Ecotoxicol Environ Saf. 2012;2012. https://doi.org/10.1016/j.ecoenv.2012.04.004.
European Commission-Pesticides Database. https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=details&pest_res_ids=302&product_ids=&v=1&e=search.pr.
Resolución-934-2010-SENASA. Servicio Nacional de Sanidad y Calidad Agroalimentaria. http://www.senasa.gob.ar/normativas/resolucion-934-2010-senasa-servicio-nacional-de-sanidad-y-calidad-agroalimentaria.
Lacroix MZ, Puel S, Toutain PL, Viguié C. Quantification of fipronil and its metabolite fipronil sulfone in rat plasma over a wide range of concentrations by LC/UV/MS. J Chromatogr B. 2010. https://doi.org/10.1016/j.jchromb.2010.05.018.
Liu Y, Zhao E, Zhu W, Gao H, Zhou Z. Determination of four heterocyclic insecticides by ionic liquid dispersive liquid–liquid microextraction in water samples. J Chromatogr A. 2009. https://doi.org/10.1016/j.chroma.2008.11.076.
Li M, Li P, Wang L, Feng M, Han L. Determination and dissipation of fipronil and its metabolites in peanut and soil. J Agric Food Chem. 2015. https://doi.org/10.1021/jf5054589.
Guo Q, Zhao S, Zhang J, Qi K, Du Z, Shao B. Determination of fipronil and its metabolites in chicken egg, muscle and cake by a modified QuEChERS method coupled with LC-MS/MS. Food Addit Contam Part A. 2018. https://doi.org/10.1080/19440049.2018.1472395.
García-Chao M, Agruña MJ, Calvete GF, Sakkas V, Llompart M, Dagnac T. Validation of an off line solid phase extraction liquid chromatography–tandem mass spectrometry method for the determination of systemic insecticide residues in honey and pollen samples collected in apiaries from NW Spain. Anal Chim Acta. 2010. https://doi.org/10.1016/j.aca.2010.03.011.
Wu J, Zhi S, Jia C, Li X, Zhu X, Zhao E. Dispersive solid-phase extraction combined with dispersive liquid-liquid microextraction for simultaneous determination of seven succinate dehydrogenase inhibitor fungicides in watermelon by ultra high performance liquid chromatography with tandem mass spectrometry. J Sep Sci. 2019. https://doi.org/10.1002/jssc.201900862.
Moreno-González D, Cutillas V, Hernando MD, Alcántara-Durán J, García-Reyes JF, Molina-Díaz A. Quantitative determination of pesticide residues in specific parts of bee specimens by nanoflow liquid chromatography high resolution mass spectrometry. Sci Total Environ. 2020. https://doi.org/10.1016/j.scitotenv.2020.137005.
Li X, Li H, Ma W, Guo Z, Li X, Song S, Tang H, Li X, Zhang Q. Development of precise GC-EI-MS method to determine the residual fipronil and its metabolites in chicken egg. Food Chem. 2019. https://doi.org/10.1016/j.foodchem.2018.12.041.
Ramasubramanian T, Paramasivam M. Determination and dissipation of fipronil and its metabolites in/on sugarcane crop. Int J Environ Anal Chem. 2017. https://doi.org/10.1080/03067319.2017.1377519.
Aparicio-Muriana MM, Lhotská I, García-Campaña AM, Lara. A first approach using micellar electrokinetic capillary chromatography for the determination of fipronil and fipronil-sulfone in eggs. Electrophoresis. 2020. https://doi.org/10.1002/elps.201900291.
Abdallah OI, Ahmed NS. Development of a vortex-assisted dispersive liquid-liquid microextraction (VA-DLLME) and LC-MS/MS procedure for simultaneous determination of fipronil and its metabolite fipronil sulfone in tomato fruits. Food Anal Methods. 2019. https://doi.org/10.1007/s12161-019-01562-z.
Zhang M, Bian K, Zhou T, Song X, Liu Q, Meng C, He L. Determination of residual fipronil in chicken egg and muscle by LC–MS/MS. J Chromatogr B. 2016. https://doi.org/10.1016/j.jchromb.2016.01.041.
Cheng Y, Dong F, Liu X, Xu J, Meng W, Liu N, Chen Z, Tao Y, Zheng Y. Simultaneous determination of fipronil and its major metabolites in corn and soil by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Methods. 2014. https://doi.org/10.1039/C3AY41742E.
El-Deen AK, Shimizu K. Deep eutectic solvent as a novel disperser in dispersive liquid-liquid microextraction based on solidification of floating organic droplet (DLLME-SFOD) for preconcentration of steroids in water samples: assessment of the method deleterious impact on the environment using analytical eco-scale and green analytical procedure index. Microchem J. 2019. https://doi.org/10.1016/j.microc.2019.103988.
Werner J. Novel deep eutectic solvent-based ultrasounds-assisted dispersive liquid-liquid microextraction with solidification of the aqueous phase for HPLC-UV determination of aromatic amines in environmental samples. Microchem J. 2020. https://doi.org/10.1016/j.microc.2019.104405.
Mogaddam MRA, Nemati M, Farajzadeh MA, Lotfipour F, Nabil AAA, Mohebbi A, Ghorbanpour H. Application of natural deep eutectic solvents-based in-syringe dispersive liquid-liquid microextraction for the extraction of five acaricides in egg samples. Int J Environ Anal Chem. 2020. https://doi.org/10.1080/03067319.2020.1774568.
Mansour FR, Danielson ND. Solidification of floating organic droplet in dispersive liquid-liquid microextraction as a green analytical tool. Talanta. 2017. https://doi.org/10.1016/j.talanta.2017.03.084.
Dai Y, Spronsen JV, Witkamp GJ, Verpoorte R, Choi YH. Natural deep eutectic solvents as a new potential media for green technology. Anal Chim Acta. 2013. https://doi.org/10.1016/j.aca.2012.12.019.
Pisano P, Espino M, Fernández MA, Silva MF, Olivieri A. Structural analysis of natural deep eutectic solvents. Theoretical and experimental study. Microchem. J. 2018. https://doi.org/10.1016/j.microc.2018.08.016.
Guiñez M, Martinez LD, Fernandez L, Cerutti S. Dispersive liquid–liquid microextraction based on solidification of floating organic drop and fluorescence detection for the determination of nitrated polycyclic aromatic hydrocarbons in aqueous samples. Microchem. 2017. https://doi.org/10.1016/j.microc.2016.10.020.
Leong MI, Huang SD. Dispersive liquid–liquid microextraction method based on solidification of floating organic drop for extraction of organochlorine pesticides in water samples. J Chromatogr A. 2009. https://doi.org/10.1016/j.chroma.2009.09.004.
Vera-Avila LE, Rojo-Portillo T, Covarrubias-Herrera R, Peña-Alvarez A. Capabilities and limitations of dispersive liquid–liquid microextraction with solidification of floating organic drop for the extraction of organic pollutants from water samples. Anal Chim Acta. 2013. https://doi.org/10.1016/j.aca.2013.10.052.
Zhang Y, Lee HK. Low-density solvent-based vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction combined with gas chromatography–mass spectrometry for the fast determination of phthalate esters in bottled water. J Chromatogr A. 2013. https://doi.org/10.1016/j.chroma.2012.12.017.
Hou X, Xu X, Xu X, Han M, Qiu S. Application of a multiclass screening method for veterinary drugs and pesticides using HPLC-QTOF-MS in egg samples. Food Chem. 2020. https://doi.org/10.1016/j.foodchem.2019.125746.
Rong L, Wu X, Xu J, Dong F, Liu X, Pan X, Du P, Wei D, Zheng Y. Simultaneous determination of three pesticides and their metabolites in unprocessed foods using ultraperformance liquid chromatography-tandem mass spectrometry. Food Add Cont: Part A. 2017. https://doi.org/10.1080/19440049.2017.1398419.
Reg. (EU) 2019/1792. Commission Regulation (EU) 2019/1792 of 17 October 2019 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for amitrole, fipronil, flupyrsulfuron-methyl, imazosulfuron, isoproturon, orthosulfamuron and triasulfuron in or on certain products. Off J European Union. L277/66.
Reg. (EU) 2016/156. Commission Regulation (EU) 2016/156 of 18 January 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for boscalid, clothianidin, thiamethoxam, folpet and tolclofos-methyl in or on certain products. Off J European Union. L31/1.
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE-Analytical GREEnness metric approach and software. Anal Chem. 2020; https://doi.org/10.1021/acs.analchem.0c01887.
Cardoso Marube L, Souza Caldas S, Lotz Soares K, Primel EG. Dispersive liquid-liquid microextraction with solidification of floating organic droplets for simultaneous extraction of pesticides, pharmaceuticals and personal care products. Microchim Acta. 2015. https://doi.org/10.1007/s00604-015-1507-7.
Acknowledgements
The authors would like to acknowledge the partial financial support to this project from University of Granada, CONICET, FONCyT, and Universidad Nacional de Cuyo. LCR thanks the Mobility Program between Andalusian and Iberoamerican Universities from the Iberoamerican Association of Postgraduate Universities (AUIP, 2019). The authors extend their appreciation to Spanish Ministry of Science, Innovation and Universities for granting the Spanish Network of Excellence in Sample preparation (RED2018-102522-T). This article is based upon work from the Sample Preparation Task Force and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Carbonell-Rozas, L., Canales, R., Lara, F.J. et al. A natural deep eutectic solvent as a novel dispersive solvent in dispersive liquid-liquid microextraction based on solidification of floating organic droplet for the determination of pesticide residues. Anal Bioanal Chem 413, 6413–6424 (2021). https://doi.org/10.1007/s00216-021-03605-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03605-z