Abstract
A sensitive and effective strategy for the detection of cytochrome c (Cyt c) and trypsin was developed using biomass nitrogen-doped carbon quantum dots (N-CQDs) as the fluorescence probe. N-CQDs were synthesized through a one-pot hydrothermal method by utilizing cellulolytic enzyme lignin as the carbon source and ammonia as the solvent and nitrogen source. The obtained N-CQDs had good water solubility and stable optical properties. The introduction of nitrogen increased fluorescence quantum yield (QY) to 8.23%, which was almost four times as high as that before nitrogen doping. The N-CQDs were fabricated as a label-free biosensor to detect Cyt c and trypsin. The fluorescence of N-CQDs was quenched with positively charged Cyt c due to electrostatic induction aggregation and static quenching. However, Cyt c tended to be hydrolyzed into small peptides in the presence of trypsin, which caused fluorescence recovery of the N-CQDs/Cyt c complex. A wide linear response range was achieved for Cyt c within 1–50 μM and the developed N-CQDs/Cyt c complex displayed a linear response for trypsin within 0.09–5.4 U/mL. The detection limits were 0.29 μM for Cyt c and 0.013 U/mL for trypsin, respectively. Furthermore, this assay had been applied to Cyt c and trypsin detection in serum samples with the recoveries in the range of 94.6–98.5% and 95.5–102.0%, respectively. The established method was sensitive, selective, easy to operate, and low cost, which proved its potential application in clinical diagnosis.
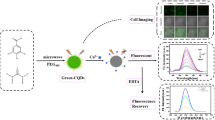
Graphical abstract

The synthesis and fluorescence mechanism of N-CQDs and the strategy for Cyt c and trypsin detection






Similar content being viewed by others
References
Lei Z, Jian M, Li X, Wei J, Meng X, Wang Z. Biosensors and bioassays for determination of matrix metalloproteinases: state of the art and recent advances. J Mater Chem B. 2020;8:3261–91.
Martinou JC, Desagher S, Antonsson B. Cytochrome c release from mitochondria: all or nothing. Nat Cell Biol. 2000;2:e41–3.
Matsuura K, Canfield K, Feng W, Kurokawa M. Metabolic regulation of apoptosis in cancer. Int Rev Cell Mol Biol. 2016;327:43–87.
Mesgari F, Beigi SM, Fakhri N, Hosseini M, Aghazadeh M, Ganjali MR. Paper-based chemiluminescence and colorimetric detection of cytochrome c by cobalt hydroxide decorated mesoporous carbon. Microchem J. 2020;157:104991.
Liu Y, Zhang F, Xu H, Ma P, Huang Y, Tao S, et al. A novel and simple fluorescent sensor based on AgInZnS QDs for the detection of protamine and trypsin and imaging of cells. Sensor Actuat B-Chem. 2019;294:263–9.
Mao G, Peng W, Tian S, Zheng J, Ji X, He Z. Dual-protein visual detection using ratiometric fluorescent probe based on Rox-DNA functionalized CdZnTeS QDs. Sensor Actuat B-Chem. 2018;283:755–60.
Zhang S, Chen C, Qin X, Zhang Q, Liu J, Zhu J, et al. Ultrasensitive detection of trypsin activity and inhibitor screening based on the electron transfer between phosphorescence copper nanocluster and cytochrome c. Talanta. 2018;189:92–9.
Tabish TA, Pranjol MZI, Karadag I, Horsell DW, Whatmore JL, Zhang S. Influence of luminescent graphene quantum dots on trypsin activity. Int J Nanomedicine. 2018;13:1525–37.
Kang OH, Jeong HJ, Kim DK, Choi SC, Kim TH, Nah YH, et al. Trypsin induces tumour necrosis factor-α secretion from a human leukemic mast cell line. Cell Biochem Funct. 2003;21:161–7.
Gaber A, Johansson M, Stenman UH, Hotakainen K, Pontén F, Glimelius B, et al. High expression of tumour-associated trypsin inhibitor correlates with liver metastasis and poor prognosis in colorectal cancer. Brit J Cancer. 2009;100:1540–8.
Artigas JM, Garcia ME, Faure MR, Gimeno AM. Serum trypsin levels in acute pancreatic and non-pancreatic abdominal conditions. Postgrad Med J. 1981;57:219–22.
Pandiaraj M, Sethy NK, Bhargava K, Kameswararao V, Karunakaran C. Designing label-free electrochemical immunosensors for cytochrome c using nanocomposites functionalized screen printed electrodes. Biosens Bioelectron. 2014;54:115–21.
Hu Q, Bao Y, Gan S, Zhang Y, Han D, Niu L. Electrochemically controlled grafting of polymers for ultrasensitive electrochemical assay of trypsin activity. Biosens Bioelectron. 2020;165:112358.
Ionescu RE, Cosnier S, Marks RS. Protease amperometric sensor. Anal Chem. 2006;78:6327–31.
Vestling MM, Murphy CM, Fenselau C. Recognition of trypsin autolysis products by high-performance liquid chromatography and mass spectrometry. Anal Chem. 1990;62:2391–4.
Zhang XM, Qin YP, Ye HL, Ma XT, He XW, Li WY, et al. Silicon nanoparticles coated with an epitope-imprinted polymer for fluorometric determination of cytochrome c. Microchim Acta. 2018;185:173.
Wang GL, Jin LY, Dong YM, Wu XM, Li ZJ. Intrinsic enzyme mimicking activity of gold nanoclusters upon visible light triggering and its application for colorimetric trypsin detection. Biosens Bioelectron. 2015;64:523–9.
Zhang W, He XW, Chen Y, Li WY, Zhang YK. Composite of CdTe quantum dots and molecularly imprinted polymer as a sensing material for cytochrome c. Biosens Bioelectron. 2011;26:2553–8.
Guo T, Deng Q, Fang G, Liu C, Huang X, Wang S. Molecularly imprinted upconversion nanoparticles for highly selective and sensitive sensing of cytochrome c. Biosens Bioelectron. 2015;74:498–503.
Gu X, Yang G, Zhang G, Zhang D, Zhu D. A new fluorescence turn-on assay for trypsin and inhibitor screening based on graphene oxide. ACS Appl Mater Interfaces. 2011;3:1175–9.
Tang B, Yang Y, Wang G, Yao Z, Zhang L, Wu HC. A simple fluorescent probe based on a pyrene derivative for rapid detection of protamine and monitoring of trypsin activity. Org Biomol Chem. 2015;13:8708–12.
Feng T, Tao S, Yue D, Zeng Q, Chen W, Yang B. Recent advances in energy conversion applications of carbon dots: from optoelectronic devices to electrocatalysis. Small. 2020;16:2001295.
Cao L, Fernando KAS, Liang W, Seilkop A, Veca LM, Sun YP, et al. Carbon dots for energy conversion applications. J Appl Phys. 2019;125:220903.
Gao X, Du C, Zhuang Z, Chen W. Carbon quantum dot-based nanoprobes for metal ion detection. J Mater Chem C. 2016;4:6927–45.
Peng J, Gao W, Gupta BK, Liu Z, Rebeca RA, Ge L, et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012;12:844–9.
Li X, Zhu S, Xu B, Ma K, Zhang J, Yang B, et al. Self-assembled graphene quantum dots induced by cytochrome c: a novel biosensor for trypsin with remarkable fluorescence enhancement. Nanoscale. 2013;5:7776–9.
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126:12736–7.
Liu R, Huang H, Li H, Liu Y, Zhong J, Li Y, et al. Metal nanoparticle/carbon quantum dot composite as a photocatalyst for high-efficiency cyclohexane oxidation. ACS Catal. 2014;4:328–36.
Ye R, Xiang C. Lin Jian, Peng Z, Huang K, Yan Z, Cook NP, Samuel ELG, Hwang CC, Ruan G, Ceriotti G, Raji ARO, Martí AA. Tour JM Coal as an abundant source of graphene quantum dots Nat Commun. 2013;4:2943.
Huang S, Yang E, Yao J, Liu Y, Xiao Q. Carbon dots doped with nitrogen and boron as ultrasensitive fluorescent probes for determination of α-glucosidase activity and its inhibitors in water samples and living cells. Microchim Acta. 2018;185:394.
Dadkhah S, Mehdinia A, Jabbari A, Manbohi A. Rapid and sensitive fluorescence and smartphone dual-mode detection of dopamine based on nitrogen-boron co-doped carbon quantum dots. Microchim Acta. 2020;187:569.
Liu S, Tian J, Wang L, Zhang Y, Qin X, Luo Y, et al. Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu (II) Ions. Adv Mater. 2012;24:2037–41.
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, et al. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions. Anal Chem. 2012;84:5351–7.
Kumawat MK, Thakur M, Gurung RB, Srivastava R. Graphene quantum dots from Mangifera indica: application in near infrared bioimaging and intracellular nanothermometry. ACS Sustain Chem Eng. 2017;5:1382–91.
Qu F, Pei H, Kong R, Zhu S, Xia L. Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbon dots and MnO2 nanosheets. Talanta. 2017;165:136–42.
Niu N, Ma Z, He F, Li S, Li J, Liu S, et al. Preparation of carbon dots for cellular imaging by the molecular aggregation of cellulolytic enzyme lignin. Langmuir. 2017;33:5786–95.
Miao X, Qu D, Yang D, Nie B, Zhao Y, Fan H, et al. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv Mater. 2018;30:1704740.
Sun S, Zhang L, Jiang K, Wu A, Lin H. Toward high-efficient red emissive carbon dots: facile preparation, unique properties, and applications as multifunctional theranostic agents. Chem Mater. 2016;28:8659–68.
Atchudan R, Edison TNJI, Sethuraman MG, Lee YR. Efficient synthesis of highly fluorescent nitrogen-doped carbon dots for cell imaging using unripe fruit extract of Prunus mume. Appl Surf Sci. 2016;384:432–41.
Jiang X, Shi Y, Liu X, Wang M, Song P, Xu F, et al. Synthesis of nitrogen-doped lignin/DES carbon quantum dots as a fluorescent probe for the detection of Fe3+ ions. Polymers-Basel. 2018;10:1282.
Li LL, Ji J, Fei R, Wang CZ, Lu Q, Zhang JR, et al. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv Funct Mater. 2012;22:2971–9.
Zhang Q, Li W, Chen J, Wang F, Wang Y, Chen Y, et al. An ultrasensitive chemiluminescence turn-on assay for protease and inhibitor screening with a natural substrate. Chem Commun. 2013;49:3137–9.
Wang S, Cole IS, Zhao D, Li Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale. 2016;8:7449–58.
Li L, Yu B, You T. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (II) ions. Biosens Bioelectron. 2015;74:263–9.
Lu S, Sui L, Liu J, Zhu S, Chen A, Jin M, et al. Near-infrared photoluminescent polymer-carbon nanodots with two-photon fluorescence. Adv Mater. 2017;29:1603443.
Ross PD, Subramanisn S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981;20:3096–102.
Zhang L, Du J. A sensitive and label-free trypsin colorimetric sensor with cytochrome c as a substrate. Biosens Bioelectron. 2016;79:346–52.
Funding
This project was funded by Natural Science Foundation of Heilongjiang Province (B2008001), Heilongjiang Postdoctoral Fund (LBH-Z16009), 111 Project (B20088), Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team), and China Postdoctoral Science Foundation (2016M591501, 2017T100218).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All applicable international, national, and/or institutional guidelines for the collection and use of human blood and serum samples were followed. All biological experiments were approved by the Ethics Committee of Harbin Medical University Cancer Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 10964 kb)
Rights and permissions
About this article
Cite this article
Yin, C., Chen, L. & Niu, N. Nitrogen-doped carbon quantum dots fabricated from cellulolytic enzyme lignin and its application to the determination of cytochrome c and trypsin. Anal Bioanal Chem 413, 5239–5249 (2021). https://doi.org/10.1007/s00216-021-03496-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03496-0