Abstract
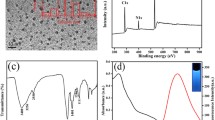
A method utilizing nitrogen-doped and sulfur-doped carbon quantum dots (N, S-CQDs) as fluorescent probes for the rapid detection of Fe3+, L-ascorbic acid (AA), and alkaline phosphatase (ALP) was presented. The fluorescence intensity of N, S-CQDs nanoprobes can be rapidly and efficiently quenched by Fe3+ and based on the fluorescence “turn off-on” characteristic of N, S-CQDs nanoprobes, the fluorescence signals of the N, S-CQDs/Fe3+can be recovered after the addition of AA. By coupling a fluorescent nanoprobe to an enzyme and L-ascorbic acid-2-phosphate (AA2P), a green, simple, rapid and effective fluorescent analytical method for the determination of ALP was developed. The prepared N, S-CQDs showed high sensitivity and selectivity to Fe3+, AA and ALP with the detection limit of 0.42 μM, 12.7 nM and 0.017 U·L−1 and their optimal concentration ranges were10—600 µM, 10—200 μM, 0.18—54 U·L−1, respectively. The fluorescence quantum yield of N, S-CQDs (0.2 mg·mL−1) at 393 nm excitation wavelength was 4.41%. Additionally, the fluorescent nanoprobes have been employed to successfully measure ALP in serum samples. It is expected that the established method may offer a new approach for biomolecular detection in clinical diagnosis and pharmaceutical analysis.













Similar content being viewed by others
Data Availability
Data available on request from the authors.
References
Sawicki KT, De Jesus A, Ardehali H (2023) Iron metabolism in cardiovascular disease: physiology, mechanisms, and therapeutic targets. Cir Res 132:379–396. https://doi.org/10.1161/CIRCRESAHA.122.321667
Senol N, Sahin M (2023) Protective Effect of Juglone (5-Hydroxy-1,4-naphthoquinone) against Iron- and Zinc-Induced Liver and Kidney Damage. Appl Sci 13. https://doi.org/10.3390/app13042203
Huang QT, Li YL (2022) Research Progress of Abnormal Iron Metabolism and Tumor of Lymphatic Hematopoiesis System --Review. Zhongguo shi yan xue ye xue za zhi 30:1277–1280. https://doi.org/10.19746/j.cnki.issn.1009-2137.2022.04.049
Kermeur N, Pedrot M, Cabello-Hurtado F (2023) Iron Availability and Homeostasis in Plants: A Review of Responses, Adaptive Mechanisms, and Signaling, Methods in molecular biology (Clifton, N.J.) 2642:49–81. https://doi.org/10.1007/978-1-0716-3044-0_3
Lazarowski AJ, Vitale AA, Auzmendi JA, Pomilio AB (2022) Iron: from normal homeostasis to death by ferroptosis. Acta Bioquimica Clinica Latinoamericana 56:491–513
Tabur S, Bayraktar NB, Ozmen S (2022) L-Ascorbic acid modulates the cytotoxic and genotoxic effects of salinity in barley meristem cells by regulating mitotic activity and chromosomal aberrations. Caryologia 75:19–29. https://doi.org/10.36253/caryologia-1791
Mokrzynski K, Krzysztynska-Kuleta O, Sarna M, Wnuk D, Wojtala M, Sarna T (2022) Skin phototoxicity of ambient particulate matter mitigated by the protective effects of ascorbic acid and resveratrol. FEBS Open Bio 12:155–155
Kola A, Nencioni F, Valensin D (2023) Bioinorganic chemistry of micronutrients related to alzheimer's and parkinson's diseases. Molecules 28. https://doi.org/10.3390/molecules28145467
Alhusaini AM, Fadda LM, Alsharafi H, Alshamary AF, Hasan IH (2022) L-Ascorbic Acid and Curcumin Prevents Brain Damage Induced via Lead Acetate in Rats: Possible Mechanisms. Dev Neurosci 44:59–66. https://doi.org/10.1159/000521619
Samie KA, Dayer D, Eshkiki ZS (2023) Human Colon Cancer HT29 Cell Line Treatment with High-Dose L-Ascorbic Acid Results to Reduced Angiogenic Proteins Expression and Elevated Pro-apoptotic Proteins Expression. Curr Mol Med 23:470–478. https://doi.org/10.2174/1566524022666220616141725
Marsalka A, Kalnaityte A, Bieksa T, Bagdonas S (2022) The combined effects of ascorbic acid and bovine serum albumin on phototransformations of hematoporphyrin derivative in aqueous medium: absorption and epr spectroscopy study, lithuanian journal of physics 62:58–71
Long Y, Yi C, Wu R, Zhang Y, Zhang B, Shi X, Zhang X, Zha Z (2023) Biodistribution and radiation dosimetry in cancer patients of the ascorbic acid analogue 6-Deoxy-6-[18F] fluoro-L-ascorbic acid PET imaging: first-in-human study. Eur J Nucl Med Mol Imaging 50:3072–3083. https://doi.org/10.1007/s00259-023-06262-9
Kumar P, Sarkar A, Jain P (2022) Green synthesis of reduced graphene oxide nanosheet by using L-ascorbic acid and study of its cytotoxicity on human cervical Cancer Cell Line. J Polym Mater 39:121–135. https://doi.org/10.32381/JPM.2022.39.1-2.8
Zhang Z, Qin S, Wang Y, Liang H, Wang R, Li F (2023) L-ascorbic acid could ameliorate the damage of myocardial microvascular endothelial cell caused by hypoxia-reoxygenation via targeting HMGB1. J Bioenerg Biomembr. https://doi.org/10.1007/s10863-023-09962-x
Makris K, Mousa C, Cavalier E (2023) Alkaline Phosphatases: Biochemistry Functions, and Measurement. Calcif Tissue Int 112:233–242. https://doi.org/10.1007/s00223-022-01048-x
Lei JS, Kang J, Liu JF, Wang GN (2022) A Novel Electrochemical Sensing Strategy Based on Poly (3, 4-ethylenedioxythiophene): Polystyrene Sulfonate, AuNPs, and Ag+ for Highly Sensitive Detection of Alkaline Phosphatase. Nanomaterials 12. https://doi.org/10.3390/nano12193392
Dharmarpandi G, Mohammed B, Al-Bayati M, Anees M, Sleem M, Alhussain EM, Naguib T (2023) Elevated alkaline phosphatase caused by isolated bone marrow breast cancer metastasis. Am J Med Sci 365:S114–S115
Mishra S, Mishra D, Mahajan B, Mantan M, Khan AM (2023) Effect of daily vitamin D supplementation on serum vitamin D levels in children with epilepsy receiving sodium valproate monotherapy: a randomized, controlled trial. Indian J Pediatr 90:450–456. https://doi.org/10.1007/s12098-022-04225-w
Haarhaus M, Cianciolo G, Barbuto S, La Manna G, Gasperoni L, Tripepi G, Plebani M, Fusaro M, Magnusson P (2022) Alkaline phosphatase: an old friend as treatment target for cardiovascular and mineral bone disorders in chronic kidney disease. Nutrients 14. https://doi.org/10.3390/nu14102124
Tse G, Zhang Q, Adhikari G, Cheung B, Basit J, Zhou J, Wong WT, Liu T, Chou O, Chung CT, Bin Waleed K, Lee S, Wong I (2023) Alkaline phosphatase variability and heart failure in type 2 diabetes mellitus: the hong kong diabetes study. Heart 109:A234–A235. https://doi.org/10.1136/heartjnl-2023-BCS.199
Diamantis D, Tsiailanis AD, Papaemmanouil C, Nika MC, Kanaki Z, Grdadolnik SG, Babic A, Tzakos EP, Fournier I, Salzet M, Kushwaha PP, Thomaidis NS, Rampias T, Shankar E, Karakurt S, Gupta S, Tzakos AG (2023) Development of a novel apigenin prodrug programmed for alkaline-phosphatase instructed self-inhibition to combat cancer. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2023.2247083
Abd Elhaleem SM, Elsebaei F, Shalan S, Belal F (2022) Turn-off fluorescence of nitrogen and sulfur carbon quantum dots as effective fluorescent probes for determination of imatinib. Application to biological fluids, Spectrochimica acta. Part A, Molecular and Miomolecular Spectroscopy 272:120954. https://doi.org/10.1016/j.saa.2022.120954
Yang Z, Xu T, Zhang X, Li H, Jia X, Zhao S, Yang Z, Liu X (2022) Nitrogen-doped carbon quantum dots as fluorescent nanosensor for selective determination and cellular imaging of ClO-. Spectrochim Acta A Mol Biomol Spectrosc 271. https://doi.org/10.1016/j.saa.2022.120941
Du Y, Li Y, Liu Y, Liu N, Cheng Y, Shi Q, Liu X, Tao Z, Guo Y, Zhang J, Askaria N, Li H (2023) Stalk-derived carbon dots as nanosensors for Fe3+ ions detection and biological cell imaging. Front Bioeng Biotechnol 11. https://doi.org/10.3389/fbioe.2023.1187632
Abd Elhaleem SM, Shalan S, Belal F, Elsebaei F (2022) Insights for applying N,S-doped carbon dots as a fluorescent nanoprobe for estimation of some nitro-calcium channel blockers. R Soc Open Sci 9:220609. https://doi.org/10.1098/rsos.220609
Abd Elhaleem SM, Elsebaei F, Shalan S, Belal F (2022) Utilization of N,S-doped carbon dots as a fluorescent nanosensor for determination of cromolyn based on inner filter effect: application to aqueous humour. Luminescence : the journal of biological and chemical luminescence 37:713–721. https://doi.org/10.1002/bio.4212
Liao S, Huang X, Yang H, Chen X (2018) Nitrogen-doped carbon quantum dots as a fluorescent probe to detect copper ions, glutathione, and intracellular pH. Anal Bioanal Chem 410:7701–7710. https://doi.org/10.1007/s00216-018-1387-x
Zhao N, Wang Y, Hou S, Zhao L (2020) Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Mikrochim Acta 187:351. https://doi.org/10.1007/s00604-020-04328-1
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
R. Fan, J.X. Xiang, P.P. Zhou, H. Mei, Y.Y. Li, H.L. Wang, X.D. Liu, X.D. Wang, Reuse of waste Myrica rubra for green synthesis of nitrogen-doped carbon dots as an "on-off-on" fluorescent probe for Fe3+ and ascorbic acid detection, Ecotoxicology and Environmental Safety, 233 (2022). https://doi.org/10.1016/j.ecoenv.2022.113350
Zhu J, Sun S, Jiang K, Wang Y, Liu W, Lin H (2017) A highly sensitive and selective fluorimetric probe for intracellular peroxynitrite based on photoinduced electron transfer from ferrocene to carbon dots. Biosens Bioelectron 97:150–156. https://doi.org/10.1016/j.bios.2017.05.054
Pang S, Liu S (2020) Dual-emission carbon dots for ratiometric detection of Fe3+ ions and acid phosphatase. Anal Chim Acta 1105:155–161. https://doi.org/10.1016/j.aca.2020.01.033
Huangfu X, Shen Y, Yang A, Liu L, Luo W, Zhao W (2020) Synthesis of water soluble CuGaS2/ZnS quantum dots for ultrasensitive fluorescent detection of alkaline phosphatase based on inner filter effect. Colloids and Surfaces B-biointerfaces 191. https://doi.org/10.1016/j.colsurfb.2020.110984
Xiao Q, Mu PP, Ning G, Zhang WQ, Li B, Huang S (2023) A ratiometric fluorescent probe for simultaneous detection of L-ascorbic acid and alkaline phosphatase activity based on red carbon dots/polydopamine nanocomposite. Talanta 264. https://doi.org/10.1016/j.talanta.2023.124724
Tang M, Zhu B, Wang Y, Wu H, Chai F, Qu F, Su Z (2019) Nitrogen- and sulfur-doped carbon dots as peroxidase mimetics: colorimetric determination of hydrogen peroxide and glutathione, and fluorimetric determination of lead(II). Mikrochim Acta 186:604. https://doi.org/10.1007/s00604-019-3710-4
Yan JY, Zhou YH, Shen JL, Zhang NN, Liu X (2023) Facile synthesis of S, N-co-doped carbon dots for bio-imaging, Fe3+detection and DFT calculation. Spectrochim Acta A Mol Biomol Spectrosc 302. https://doi.org/10.1016/j.saa.2023.123105
Sun LL, Liu YY, Wang YS, Xu JY, Xiong Z, Zhao XH, Xia YZ (2021) Nitrogen and sulfur Co-doped carbon dots as selective and visual sensors for monitoring cobalt ions. Opt Mater 112. https://doi.org/10.1016/j.optmat.2020.110787
Saraswat V, Yadav M (2021) Improved corrosion resistant performance of mild steel under acid environment by novel carbon dots as green corrosion inhibitor. Colloids Surf A Physicochem Eng Asp 627. https://doi.org/10.1016/j.colsurfa.2021.127172
Rajar K, Alveroglu E (2020) CNTs based Water soluble fluorescent sensor for selective detection of Fe3+ ion. Mater Res Bull 124. https://doi.org/10.1016/j.materresbull.2019.110748
Mohandoss S, Ahmad N, Khan MR, Velu KS, Kalaiselvi K, Palanisamy S, You SG, Lee YR (2023) Multicolor emission-based nitrogen, sulfur and boron co-doped photoluminescent carbon dots for sequential sensing of Fe3+ and cysteine: RGB color sensor and live cell imaging. Spectrochim Acta A Mol Biomol Spectrosc 302. https://doi.org/10.1016/j.saa.2023.123040
Jin XL, Bai HY, Ma YT, Li YL, Chen WX (2023) Green synthesis of biomass-based fluorescent carbon dots for the detection and adsorption of Fe (III). Chemistryselect 8. https://doi.org/10.1002/slct.202204852
Tang FR, Feng ST, Wu FS, Zhang J (2023) One-step fabrication of biomass carbon dots for highly selective detection of Fe3+. Chem Pap. https://doi.org/10.1007/s11696-023-03153-z
Chen J, Wang YT, Wang L, Liu MJ, Fang LL, Chu P, Gao CZ, Chen DP, Ren DZ, Zhang JB (2023) Multi-applications of carbon dots and polydopamine-coated carbon dots for Fe3+ detection, bioimaging, dopamine assay and photothermal therapy. Discover Nano 18. https://doi.org/10.1186/s11671-023-03809-5
Wang Z, Chen D, Gu B, Gao B, Liu Z, Yang Y, Guo Q, Zheng X, Wang G (2020) Yellow emissive nitrogen-doped graphene quantum dots as a label-free fluorescent probe for Fe3+ sensing and bioimaging. Diam Relat Mater 104. https://doi.org/10.1016/j.diamond.2020.107749
Li C, Sun Q, Zhao Q, Cheng X (2020) Highly selective ratiometric fluorescent probes for the detection of Fe3+ and its application in living cells. Spectrochim Acta A Mol Biomol Spectrosc 228. https://doi.org/10.1016/j.saa.2019.117720
Chen L, Wul C, Du P, Feng X, Wu P, Cai C (2017) Electrolyzing synthesis of boron-doped graphene quantum dots for fluorescence determination of Fe3+ ions in water samples. Talanta 164:100–109. https://doi.org/10.1016/j.talanta.2016.11.019
Wang Q-B, Zhang C-J, Yu H, Zhang X, Lu Q, Yao J-S, Zhao H (2020) The sensitive “Turn-on” fluorescence platform of ascorbic acid based on conjugated polymer nanoparticles. Anal Chim Acta 1097:153–160. https://doi.org/10.1016/j.aca.2019.10.076
Xu HB, Zhou SH, Li MY, Zhang PR, Wang ZH, Tian YM, Wang XQ (2022) Preparation of biomass-waste-derived carbon dots from apricot shell for highly sensitive and selective detection of ascorbic acid. Chin J Anal Chem 50. https://doi.org/10.1016/j.cjac.2022.100168
Li JX, Liu MT, Lu CF, Xu YJ, Cao QE, Zhou CH (2023) Microwave-assisted synthesis of boron and nitrogen-doped carbon dots for detection of ascorbic acid. Chin J Anal Chem 51:211–218. https://doi.org/10.19756/j.issn.0253-3820.221314
Xu OW, Wan SY, Yang J, Song HY, Xia J, Dong LZ, Zhu XS (2023) Ionic liquid-functionalized carbon dots with positive surface charge for selective detection of ascorbic acid. Carbon Letters 33:387–395. https://doi.org/10.1007/s42823-022-00427-6
Won S, Kim J (2022) The detection of Fe (III) and ascorbic acid by fluorescence quenching and recovery of carbon dots prepared from coffee waste. Korean J Chem Eng 39:2826–2833. https://doi.org/10.1007/s11814-022-1138-8
Liu Y, Wu P, Wu X, Ma C, Luo S, Xu M, Li W, Liu S (2020) Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 210. https://doi.org/10.1016/j.talanta.2019.120649
Niu W-J, Shan D, Zhu R-H, Deng S-Y, Cosnier S, Zhang X-J (2016) Dumbbell-shaped carbon quantum dots/AuNCs nanohybrid as an efficient ratiometric fluorescent probe for sensing cadmium (II) ions and L-ascorbic acid. Carbon 96:1034–1042. https://doi.org/10.1016/j.carbon.2015.10.051
Yang Y-X, Fang Y-Z, Tian J-X, Xiao Q, Kong X-J (2020) Fluorescent polydopamine nanoparticles as a nanosensor for the sequential detection of mercury ions andl-ascorbic acid based on a coordination effect and redox reaction. RSC Adv 10:28164–28170. https://doi.org/10.1039/d0ra02031a
Lv X, Man H, Dong L, Huang J, Wang X (2020) Preparation of highly crystalline nitrogen-doped carbon dots and their application in sequential fluorescent detection of Fe3+ and ascorbic acid. Food Chem 326. https://doi.org/10.1016/j.foodchem.2020.126935
He SB, Balasubramanian P, Hu AL, Zheng XQ, Lin MT, Xiao MX, Peng HP, Deng HH, Chen W (2020) One-pot cascade catalysis at neutral pH driven by CuO tandem nanozyme for ascorbic acid and alkaline phosphatase detection. Sensors and Actuators B-Chemical 321. https://doi.org/10.1016/j.snb.2020.128511
Wang S, Han BB, Chen MS, Han YQ, Liu JF, Wang GN (2023) Construction of bifunctional carbon dots based fluorescent/colorimetric/ smartphone-assisted multi-signal strategy for monitoring alkaline phosphatase activity. Materials & Design 232. https://doi.org/10.1016/j.matdes.2023.112172
Li PH, Liang N, Liu C, Xia L, Qu FL, Song ZL, Kong RM (2022) Silver ion-regulated ratiometric fluorescence assay for alkaline phosphatase detection based on carbon dots and o-phenylenediamine. Spectrochim Acta A Mol Biomol Spectrosc 282. https://doi.org/10.1016/j.saa.2022.121682
Wu T, Hou W, Ma Z, Liu M, Liu X, Zhang Y, Yao S (2019) Colorimetric determination of ascorbic acid and the activity of alkaline phosphatase based on the inhibition of the peroxidase-like activity of citric acid-capped Prussian Blue nanocubes. Microchimica Acta 186. https://doi.org/10.1007/s00604-018-3224-5
Liu SG, Han L, Li N, Fan YZ, Yang YZ, Li NB, Luo HQ (2019) A ratiometric fluorescent strategy for alkaline phosphatase activity assay based on g-C3N4/CoOOH nanohybrid via target-triggered competitive redox reaction. Sens Actuators b Chem 283:515–523. https://doi.org/10.1016/j.snb.2018.12.052
Yang Q, Li C, Li J, Arabi M, Wang X, Peng H, Xiong H, Choo J, Chen L (2020) Multi-emitting fluorescence sensor of MnO2-OPD-QD for the multiplex and visual detection of ascorbic acid and alkaline phosphatase. J Mater Chem C 8:5554–5561. https://doi.org/10.1039/c9tc07072a
Acknowledgements
Associate professor Yang Wang, School of Pharmacy, Shenyang Pharmaceutical University, 103 Wenhua Road Shenhe District, 110016, Shenyang, Liaoning Province, P. R. China; Shandong Lukang Pharmaceutical Co., Ltd., Jining, Shandong Province, P. R. China.
Funding
This work was supported by the Middle-aged Backbone Personnel Training Program of Shenyang Pharmaceutical University (ZQN2016011) and Science and Technology Department of Liaoning Province(2019-ZD-0450).
Author information
Authors and Affiliations
Contributions
RNW: Conceptualization, Methodology, Data curation, Writing-original draft. YW and NZ: Formal analysis, Investigation, Software, Data curation. HQZ and XCY: Writing-review & Editing, Supervision. Longshan Zhao: Writing-review & Editing, Project administration.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
The human serum samples used in this study were provided by the First Affiliated Hospital of Jilin University and were approved by the Ethics Committee.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Wang, Y., Zhao, N. et al. Nitrogen and Sulfur Co-doped Carbon Quantum Dots for Detecting Fe3+, Ascorbic Acid and Alkaline Phosphatase Activities. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03539-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03539-y