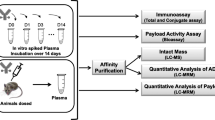
Abstract
Antibody drug conjugates (ADCs) represent a rapidly growing modality for the treatment of numerous oncology indications. The complexity of analytical characterization method development is increased due to the potential for synthetic intermediates and process-related impurities. In addition, the cytotoxicity of such materials provides an additional challenge with regard to handling products and/or sharing materials with analytical collaborators and/or vendors for technology development. Herein, we have utilized a site-specific chemoenzymatic glycoconjugation strategy for preparing ADC mimetics composed of the NIST monoclonal antibody (NISTmAb) conjugated to non-cytotoxic payloads representing both small molecules and peptides. The materials were exhaustively characterized with high-resolution mass spectrometry-based approaches to demonstrate the utility of each analytical method for confirming the conjugation fidelity as well as deep characterization of low-abundance synthetic intermediates and impurities arising from payload raw material heterogeneity. These materials therefore represent a widely available test metric to develop novel ADC analytical methods as well as a platform to discuss best practices for extensive characterization.





Similar content being viewed by others
References
Mouchahoir T, Schiel JE. Development of an LC-MS/MS peptide mapping protocol for the NISTmAb. Anal Bioanal Chem. 2018;410(8):2111–26. https://doi.org/10.1007/s00216-018-0848-6.
Schiel JE, Turner A. The NISTmAb Reference Material 8671 lifecycle management and quality plan. Anal Bioanal Chem. 2018;410(8):2067–78. https://doi.org/10.1007/s00216-017-0844-2.
Schiel JE, Turner A, Mouchahoir T, Yandrofski K, Telikepalli S, King J, et al. The NISTmAb Reference Material 8671 value assignment, homogeneity, and stability. Anal Bioanal Chem. 2018;410(8):2127–39. https://doi.org/10.1007/s00216-017-0800-1.
Turner A, Schiel JE. Qualification of NISTmAb charge heterogeneity control assays. Anal Bioanal Chem. 2018;410(8):2079–93. https://doi.org/10.1007/s00216-017-0816-6.
Turner A, Yandrofski K, Telikepalli S, King J, Heckert A, Filliben J, et al. Development of orthogonal NISTmAb size heterogeneity control methods. Anal Bioanal Chem. 2018;410(8):2095–110. https://doi.org/10.1007/s00216-017-0819-3.
Arbogast LW, Delaglio F, Schiel JE, Marino JP. Multivariate Analysis of Two-Dimensional (1)H, (13)C Methyl NMR spectra of monoclonal antibody therapeutics to facilitate assessment of higher order structure. Anal Chem. 2017;89(21):11839–45. https://doi.org/10.1021/acs.analchem.7b03571.
Brinson RG, Marino JP, Delaglio F, Arbogast LW, Evans RM, Kearsley A, et al. Enabling adoption of 2D-NMR for the higher order structure assessment of monoclonal antibody therapeutics. mAbs. 2018. https://doi.org/10.1080/19420862.2018.1544454.
Castellanos MM, Howell SC, Gallagher DT, Curtis JE. Characterization of the NISTmAb Reference Material using small-angle scattering and molecular simulation : part I: dilute protein solutions. Anal Bioanal Chem. 2018. https://doi.org/10.1007/s00216-018-0868-2.
Castellanos MM, Mattison K, Krueger S, Curtis JE. Characterization of the NISTmAb Reference Material using small-angle scattering and molecular simulation : part II: concentrated protein solutions. Anal Bioanal Chem. 2018. https://doi.org/10.1007/s00216-018-0869-1.
Cavicchi RE, King J, Ripple DC. Measurement of average aggregate density by sedimentation and Brownian motion analysis. J Pharm Sci. 2018;107(5):1304–12. https://doi.org/10.1016/j.xphs.2018.01.013.
Dong Q, Liang Y, Yan X, Markey SP, Mirokhin YA, Tchekhovskoi DV, et al. The NISTmAb tryptic peptide spectral library for monoclonal antibody characterization. mAbs. 2018;10(3):354–69. https://doi.org/10.1080/19420862.2018.1436921.
Dong Q, Yan X, Liang Y, Stein SE. In-depth characterization and spectral library building of glycopeptides in the tryptic digest of a monoclonal antibody using 1D and 2D LC-MS/MS. J Proteome Res. 2016;15(5):1472–86. https://doi.org/10.1021/acs.jproteome.5b01046.
Gallagher DT, Karageorgos I, Hudgens JW, Galvin CV. Data on crystal organization in the structure of the Fab fragment from the NIST reference antibody, RM 8671. Data in brief. 2018;16:29–36. https://doi.org/10.1016/j.dib.2017.11.013.
Hill JJ, Tremblay TL, Corbeil CR, Purisima EO, Sulea T. An accurate TMT-based approach to quantify and model lysine susceptibility to conjugation via N-hydroxysuccinimide esters in a monoclonal antibody. Sci Rep. 2018;8(1):17680. https://doi.org/10.1038/s41598-018-35924-0.
Hilliard M, Alley WR Jr, McManus CA, Yu YQ, Hallinan S, Gebler J, et al. Glycan characterization of the NIST RM monoclonal antibody using a total analytical solution: from sample preparation to data analysis. mAbs. 2017;9(8):1349–59. https://doi.org/10.1080/19420862.2017.1377381.
Kalonia CK, Heinrich F, Curtis JE, Raman S, Miller MA, Hudson SD. Protein adsorption and layer formation at the stainless steel-solution interface mediates shear-induced particle formation for an IgG1 monoclonal antibody. Mol Pharm. 2018;15(3):1319–31. https://doi.org/10.1021/acs.molpharmaceut.7b01127.
Karageorgos I, Gallagher ES, Galvin C, Gallagher DT, Hudgens JW. Biophysical characterization and structure of the Fab fragment from the NIST reference antibody, RM 8671. Biologicals. 2017;50:27–34. https://doi.org/10.1016/j.biologicals.2017.09.005.
Schiel JE, Davis DL, Borisov OB (eds) (2015) State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization volume 3. Defining the next generation of analytical and biophysical techniques, vol 1202. ACS Symposium Series, vol 1202. American Chemical Society. doi:https://doi.org/10.1021/bk-2015-1202
Schiel JE, Davis DL, Borisov OB (eds) (2015) State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study, vol 1201. ACS Symposium Series, vol 1201. American Chemical Society. doi:https://doi.org/10.1021/bk-2015-1201
van der Burgt YEM, Kilgour DPA, Tsybin YO, Srzentic K, Fornelli L, Beck A, et al. Structural analysis of monoclonal antibodies by ultra-high resolution MALDI In-Source Decay FT-ICR mass spectrometry. Anal Chem. 2018. https://doi.org/10.1021/acs.analchem.8b04515.
Singh SK, Luisi DL, Pak RH. Antibody-drug conjugates: design, formulation and physicochemical stability. Pharm Res. 2015;32(11):3541–71. https://doi.org/10.1007/s11095-015-1704-4.
Rathore D, Faustino A, Schiel J, Pang E, Boyne M, Rogstad S. The role of mass spectrometry in the characterization of biologic protein products. Expert Rev Proteomics. 2018;15(5):431–49. https://doi.org/10.1080/14789450.2018.1469982.
Jain N, Smith SW, Ghone S, Tomczuk B. Current ADC linker chemistry. Pharm Res. 2015;32(11):3526–40. https://doi.org/10.1007/s11095-015-1657-7.
Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M, Gentile R, et al. Antibody-drug conjugates: the new frontier of chemotherapy. Intl. J Pharm Sci. 2020;21(15). https://doi.org/10.3390/ijms21155510.
Sadiki A, Vaidya SR, Abdollahi M, Bhardwaj G, Dolan ME, Turna H, et al. Site-specific conjugation of native antibody. Antibody therapeutics. 2020;3(4):271–84. https://doi.org/10.1093/abt/tbaa027.
Valliere-Douglass JF, McFee WA, Salas-Solano O. Native intact mass determination of antibodies conjugated with monomethyl Auristatin E and F at interchain cysteine residues. Anal Chem. 2012;84(6):2843–9. https://doi.org/10.1021/ac203346c.
Beckley NS, Lazzareschi KP, Chih HW, Sharma VK, Flores HL. Investigation into temperature-induced aggregation of an antibody drug conjugate. Bioconjug Chem. 2013;24(10):1674–83. https://doi.org/10.1021/bc400182x.
Guo J, Kumar S, Prashad A, Starkey J, Singh SK. Assessment of physical stability of an antibody drug conjugate by higher order structure analysis: impact of thiol- maleimide chemistry. Pharm Res. 2014;31(7):1710–23. https://doi.org/10.1007/s11095-013-1274-2.
Prien JM, Stöckmann H, Albrecht S, Martin SM, Varatta M, Furtado M, Hosselet S, Wang M, Formolo T, Rudd PM, Schiel JE Orthogonal technologies for NISTmAb N-glycan structure elucidation and quantitation. In: State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study, vol 1201. ACS Symposium Series, vol 1201. American Chemical Society. 2015; pp 185-235. doi:https://doi.org/10.1021/bk-2015-1201.ch004
Sjögren J, Cosgrave EF, Allhorn M, Nordgren M, Björk S, Olsson F, et al. EndoS and EndoS2 hydrolyze Fc-glycans on therapeutic antibodies with different glycoform selectivity and can be used for rapid quantification of high-mannose glycans. Glycobiology. 2015;25(10):1053–63. https://doi.org/10.1093/glycob/cwv047.
Qasba PK. Glycans of antibodies as a specific site for drug conjugation using glycosyltransferases. Bioconjug Chem. 2015;26(11):2170–5. https://doi.org/10.1021/acs.bioconjchem.5b00173.
Li W, Kerwin JL, Schiel J, Formolo T, Davis D, Mahan A, Benchaar SA. Structural elucidation of post-translational modifications in monoclonal antibodies. In: State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study, vol 1201. ACS Symposium Series, vol 1201. American Chemical Society. 2015; pp 119-183. doi:https://doi.org/10.1021/bk-2015-1201.ch003
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
NIST Disclaimer: Values reported herein do not supersede official NISTmAb Report of Investigation and are for informational purposes only. Users should always refer to the Report of Investigation (https://www-s.nist.gov/srmors/view_detail.cfm?srm=8671) for their specific material lot for the most up to date values and uncertainty ranges. Certain commercial equipment, instruments, or materials are identified to adequately specify the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Supplementary Information
ESM 1
(DOCX 231 kb)
Rights and permissions
About this article
Cite this article
Agnew, B., Lin, S., Zhang, T. et al. Site-specific glycan-conjugated NISTmAb antibody drug conjugate mimetics: synthesis, characterization, and utility. Anal Bioanal Chem 413, 4989–5001 (2021). https://doi.org/10.1007/s00216-021-03460-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03460-y