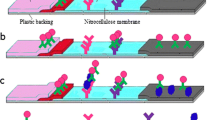
Abstract
Listeria monocytogenes is an invasive opportunistic foodborne pathogen and its routine surveillance is critical for protecting the food supply and public health. The traditional detection methods are time-consuming and require trained personnel. Lateral flow immunoassay (LFIA), on the other hand, is an easy-to-perform, rapid point-of-care test and has been widely used as an inexpensive surveillance tool. In recent times, nucleic acid–based lateral flow immunoassays (NALFIA) are also developed to improve sensitivity and specificity. A significant improvement in lateral flow–based assays has been reported in recent years, especially the ligands (antibodies, nucleic acids, aptamers, bacteriophage), labeling molecules, and overall assay configurations to improve detection sensitivity, specificity, and automated interpretation of results. In most commercial applications, LFIA has been used with enriched food/environmental samples to ensure detection of live cells thus prolonging the assay time to 24–48 h; however, with the recent improvement in LFIA sensitivity, results can be obtained in less than 8 h with shortened and improved enrichment practices. Incorporation of surface-enhanced Raman spectroscopy and/or immunomagnetic separation could significantly improve LFIA sensitivity for near-real-time point-of-care detection of L. monocytogenes for food safety and public health applications.





Similar content being viewed by others
References
Drolia R, Bhunia AK. Crossing the intestinal barrier via Listeria adhesion protein and internalin A. Trends Microbiol. 2019;27(5):408–25.
Bhunia AK. Listeria monocytogenes. In: Bhunia AK, editor. Foodborne microbial pathogens: mechanisms and pathogenesis. New York: Springer; 2018. p. 229–48.
Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16(1):32–46.
Leclercq A, Moura A, Vales G, Tessaud-Rita N, Aguilhon C, Lecuit M. Listeria thailandensis sp. nov. Int J Syst Evol Microbiol. 2019;69(1):74–81.
Núñez-Montero K, Leclercq A, Moura A, Vales G, Peraza J, Pizarro-Cerdá J, et al. Listeria costaricensis sp. nov. Int J Syst Evol Microbiol. 2018;68(3):844–50.
Chen J-Q, Healey S, Regan P, Laksanalamai P, Hu Z. PCR-based methodologies for detection and characterization of Listeria monocytogenes and Listeria ivanovii in foods and environmental sources. Food Sci Human Wellness. 2017;6(2):39–59.
Weller D, Andrus A, Wiedmann M, den Bakker HC. Listeria booriae sp. nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Int J Syst Evol Microbiol. 2015;65(1):286–92.
de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(11):1073–82.
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7–15.
Hoffmann SA, Maculloch B, Batz M. Economic burden of major foodborne illnesses acquired in the United States. United States Department of Agriculture, Economic Research Service. 2015.
Smith AM, Tau NP, Smouse SL, Allam M, Ismail A, Ramalwa NR, et al. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog Dis. 2019;16(7):524–30.
Olanya OM, Hoshide AK, Ijabadeniyi OA, Ukuku DO, Mukhopadhyay S, Niemira BA, et al. Cost estimation of listeriosis (Listeria monocytogenes) occurrence in South Africa in 2017 and its food safety implications. Food Control. 2019;102:231–9.
Desai AN, Anyoha A, Madoff LC, Lassmann B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: a review of ProMED reports from 1996 to 2018. Int J Infect Dis. 2019;84:48–53.
Lamont RF, Sobel J, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, Kim SK, et al. Listeriosis in human pregnancy: a systematic review. J Perinat Med. 2011;39(3):227–36.
Schlech WF. Epidemiology and clinical manifestations of Listeria monocytogenes infection. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Braunstein M, Rood JI, editors. Gram-positive pathogens; 2019. p. 793–802.
Roberts BN, Chakravarty D, Gardner JC, Ricke SC, Donaldson JR. Listeria monocytogenes response to anaerobic environments. Pathogens. 2020;9(3):210.
Horn N, Bhunia AK. Food-associated stress primes foodborne pathogens for the gastrointestinal phase of infection. Front Microbiol. 2018;9:1962.
van der Veen S, Moezelaar R, Abee T, Wells-Bennik MHJ. The growth limits of a large number of Listeria monocytogenes strains at combinations of stresses show serotype- and niche-specific traits. J Appl Microbiol. 2008;105(5):1246–58.
Pérez-Ibarreche M, Castellano P, Leclercq A, Vignolo G. Control of Listeria monocytogenes biofilms on industrial surfaces by the bacteriocin-producing Lactobacillus sakei CRL1862. FEMS Microbiol Lett. 2016;363(12):1–6.
Bhunia AK. One day to one hour: how quickly can foodborne pathogens be detected? Future Microbiol. 2014;9(8):935–46.
Auvolat A, Besse NG. The challenge of enumerating Listeria monocytogenes in food. Food Microbiol. 2016;53:135–49.
Chen J-Q, Regan P, Laksanalamai P, Healey S, Hu Z. Prevalence and methodologies for detection, characterization and subtyping of Listeria monocytogenes and L. ivanovii in foods and environmental sources. Food Sci Human Wellness. 2017;6(3):97–120.
W-b S, J-g C, J-y K, Yang Z-y, Lee K-h, M-g K. Enhanced rapidity for qualitative detection of Listeria monocytogenes using an enzyme-linked immunosorbent assay and enhanced rapidity for qualitative detection of Listeria monocytogenes using an enzyme-linked immunosorbent assay and immunochromatography. J Food Prot. 2008;71(4):781–9.
Jaakohuhta S, Härmä H, Tuomola M, Lövgren T. Sensitive Listeria spp. immunoassay based on europium(III) nanoparticulate labels using time-resolved fluorescence. Int J Food Microbiol. 2007;114(3):288–94.
Banada PP, Bhunia AK. Antibodies and immunoassays for detection of bacterial pathogens. In: Zourob M, Elwary S, Turner A, editors. Principles of bacterial detection: biosensors, recognition receptors and microsystems. Manchester: Cambridge University; 2008. p. 567–602.
Davis D, Guo X, Musavi L, Lin C-S, Chen S-H, Wu VCH. Gold nanoparticle-modified carbon electrode biosensor for the detection of Listeria monocytogenes. Ind Biotechnol. 2013;9(1):31–6.
Ohk SH, Koo OK, Sen T, Yamamoto CM, Bhunia AK. Antibody-aptamer functionalized fibre-optic biosensor for specific detection of Listeria monocytogenes from food. J Appl Microbiol. 2010;109(3):808–17.
Sharma H, Mutharasan R. Rapid and sensitive immunodetection of Listeria monocytogenes in milk using a novel piezoelectric cantilever sensor. Biosens Bioelectron. 2013;45(1):158–62.
Mendonca M, Conrad N, Conceicao F, Moreira A, da Silva W, Aleixo J, et al. Highly specific fiber optic immunosensor coupled with immunomagnetic separation for detection of low levels of Listeria monocytogenes and L. ivanovii. BMC Microbiol. 2012;12(1):275.
Bhunia AK. Biosensors and bio-based methods for the separation and detection of foodborne pathogens. Adv Food Nutr Res. 2008;54:1–44.
Abdelhaseib MU, Singh AK, Bhunia AK. Simultaneous detection of Salmonella enterica, Escherichia coli and Listeria monocytogenes in food using a light scattering sensor. J Appl Microbiol. 2019;126(5):1496–507.
Xu L, Bai X, Bhunia AK. Current state of biosensors development and their application in foodborne pathogen detection. J Food Prot. 2021. https://doi.org/10.4315/JFP-4320-4464.
Martelet A, L’Hostis G, Nevers M-C, Volland H, Junot C, Becher F, et al. Phage amplification and immunomagnetic separation combined with targeted mass spectrometry for sensitive detection of viable bacteria in complex food matrices. Anal Chem. 2015;87(11):5553–60.
Richter Ł, Janczuk-Richter M, Niedziółka-Jönsson J, Paczesny J, Hołyst R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov Today. 2018;23(2):448–55.
Välimaa AL, Tilsala-Timisjärvi A, Virtanen E. Rapid detection and identification methods for Listeria monocytogenes in the food chain - a review. Food Control. 2015;55:103–14.
Gholipour S, Nikaeen M, Farhadkhani M, Nikmanesh B. Survey of Listeria monocytogenes contamination of various environmental samples and associated health risks. Food Control. 2020;108:106843.
Zhang CXY, Brooks BW, Huang H, Pagotto F, Lin M. Identification of surface protein biomarkers of Listeria monocytogenes via bioinformatics and antibody-based protein detection tools. Appl Environ Microbiol. 2016;82(17):5465–76.
Lopes-Luz L, Mendonça M, Bernardes Fogaça M, Kipnis A, Bhunia AK, Bührer-Sékula S. Listeria monocytogenes: review of pathogenesis and virulence determinants-targeted immunological assays. Crit Rev Microbiol. 2021;1–20. https://doi.org/10.1080/1040841X.1042021.1911930.
Sajid M, Kawde AN, Daud M. Designs, formats and applications of lateral flow assay: a literature review. J Saudi Chem Soc. 2015;19(6):689–705.
Law JW-F, Ab Mutalib N-S, Chan K-G, Lee L-H. An insight into the isolation, enumeration, and molecular detection of Listeria monocytogenes in food. Front Microbiol. 2015;6:1227–7.
O’Farrell B. Evolution in lateral flow–based immunoassay systems. In: Lateral flow immunoassay: Springer; 2009. p. 1–33.
Gombas DE, Chen Y, Clavero RS, Scott VN. Survey of Listeria monocytogenes in ready-to-eat foods. J Food Prot. 2003;66(4):559–69.
Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018;16(1):5134.
Luchansky JB, Chen Y, Porto-Fett AC, Pouillot R, Shoyer BA, Johnson-DeRycke R, et al. Survey for Listeria monocytogenes in and on ready-to-eat foods from retail establishments in the United States (2010 through 2013): assessing potential changes of pathogen prevalence and levels in a decade. J Food Prot. 2017;80(6):903–21.
Little CL, Sagoo SK, Gillespie IA, Grant K, McLauchlin J. Prevalence and level of Listeria monocytogenes and other Listeria species in selected retail ready-to-eat foods in the United Kingdom. J Food Prot. 2009;72(9):1869–77.
Plotz CM, Singer J. The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. Am J Med. 1956;21(6):888.
Berson SA, Yalow RS. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959;38(11):1996–2016.
Avrameas S. Coupling of enzymes to proteins with glutaraldehyde: use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969;6(1):43–52.
Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Banerjee R, Jaiswal A. Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. Analyst. 2018;143(9):1970–96.
Oliveira JP, Prado AR, Keijok WJ, Antunes PWP, Yapuchura ER, Guimarães MCC. Impact of conjugation strategies for targeting of antibodies in gold nanoparticles for ultrasensitive detection of 17β-estradiol. Sci Rep. 2019;9(1):1–8.
Huang Y, Xu T, Wang W, Wen Y, Li K, Qian L, et al. Lateral flow biosensors based on the use of micro-and nanomaterials: a review on recent developments. Microchim Acta. 2020;187(1):1–25.
Tsai T-T, Huang T-H, Chen C-A, Ho NY-J, Chou Y-J, Chen C-F. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay. Sci Rep. 2018;8(1):17319.
Urusov AE, Zherdev AV, Dzantiev BB. Towards lateral flow quantitative assays: detection approaches. Biosensors. 2019;9(3):1–25.
Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569–82.
Shan S, Lai W, Xiong Y, Wei H, Xu H. Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens : a review. J Agric Food Chem. 2015;63(3):745–53.
Hsieh HV, Dantzler JL, Weigl BH. Analytical tools to improve optimization procedures for lateral flow assays. Diagnostics (Basel). 2017;7(2):29.
Bahadır EB, Sezgintürk MK. Lateral flow assays: principles, designs and labels, vol. 82: Elsevier B.V; 2016.
Shi L, Wu F, Wen Y, Zhao F, Xiang J, Ma L. A novel method to detect Listeria monocytogenes via superparamagnetic lateral flow immunoassay. Anal Bioanal Chem. 2014;407(December):529–35.
Moyano A, Serrano-Pertierra E, Salvador M, Martínez-García JC, Rivas M, Blanco-López MC. Magnetic lateral flow immunoassays. Diagnostics. 2020;10(5):288.
Nguyen V-T, Song S, Park S, Joo C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens Bioelectron. 2020;152:112015.
Otles S, Ozyurt VH. Sampling and sample preparation. In: Handbook of food chemistry. Berlin/Heidelberg: Springer; 2015. p. 151–64.
Brehm-Stecher B, Young C, Jaykus L-A, Tortorello ML. Sample preparation: the forgotten beginning. J Food Prot. 2009;72(8):1774–89.
Li X, Ximenes E, Amalaradjou MAR, Vibbert HB, Foster K, Jones J, et al. Rapid sample processing for detection of food-borne pathogens via cross-flow microfiltration. Appl Environ Microbiol. 2013;79(22):7048.
Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Phil Trans R Soc A: Math Phys Eng Sci. 1915;2010(368):1333–83.
Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43(3):744–64.
Mahmoudi R, Norian R. Aflatoxin B1 and M1 contamination in cow feeds and milk from Iran. Food Agric Immunol. 2015;26(1):131–7.
Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J. 2005;46(3):258–68.
Wiedmann M, Wang S, Post L, Nightingale K. Assessment criteria and approaches for rapid detection methods to be used in the food industry. J Food Prot. 2014;77(4):670–90.
Postollec F, Falentin H, Pavan S, Combrisson J, Sohier D. Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiol. 2011;28(5):848–61.
European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off J Eur Union. 2005;50:1–26.
MAPA. Programa de Controle de Listeria monocytogenes em Produtos de Origem Animal Prontos para Consumo. Ministério da Agricultura, Pecuária e Abastecimento, Gov.Br. 2017. https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/controle-de-patogenos/listeria-monocytogenes. Accessed 02/03/2021.
USFDA. Release of a new report on the sources of foodborne illnesses for 2016 from the Interagency Food Safety Analytics Collaboration. US Food and Drug Administration. 2018. https://www.fda.gov/food/cfsan-constituent-updates/release-new-report-sources-foodborne-illnesses-2016-interagency-foodsafety-analytics-collaboration. Accessed 02/03/2021.
Sapountzi E, Braiek M, Chateaux JF, Jaffrezic-Renault N, Lagarde F. Recent advances in electrospun nanofiber interfaces for biosensing devices. Sensors. 2017;17(8):1887.
Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sensing Biosens Res. 2016;9:17–22.
Nayl AAA, Abd-Elhamid AII, El-Moghazy AY, Hussin M, Abu-Saied MAA, El-Shanshory AA, et al. The nanomaterials and recent progress in biosensing systems: a review. Trends Environ Anal Chem. 2020;26:e00087–7.
Faulstich K, Gruler R, Eberhard M, Lentzsch D, Haberstroh K. Handheld and portable reader devices for lateral flow immunoassays. Totowa: Humana Press; 2009. p. 1–27.
Ma S, He J, Guo M, Sun X, Zheng M, Wang Y. Ultrasensitive colorimetric detection of triazophos based on the aggregation of silver nanoparticles. Colloids Surf A Physicochem Eng Asp. 2018;538:343–9.
Gong X, Cai J, Zhang B, Zhao Q, Piao J, Peng W, et al. A review of fluorescent signal-based lateral flow immunochromatographic strips. J Mater Chem B. 2017;5(26):5079–91.
Wu Z. Simultaneous detection of Listeria monocytogenes and Salmonella Typhimurium by a SERS-based lateral flow immunochromatographic assay. Food Anal Methods. 2019;12(5):1086–91.
Taton K, Johnson D, Guire P, Lange E, Tondra M. Lateral flow immunoassay using magnetoresistive sensors. J Magnetism Magnetic Mat. 2009;321(10):1679–82.
Jacinto MJ, Trabuco JR, Vu BV, Garvey G, Khodadady M, Azevedo AM, et al. Enhancement of lateral flow assay performance by electromagnetic relocation of reporter particles. PLoS One. 2018;13(1):e0186782.
Tominaga T. Enhanced sensitivity of lateral-flow test strip immunoassays using colloidal palladium nanoparticles and horseradish peroxidase. LWT. 2017;86:566–70.
Cho I-H, Irudayaraj J. Lateral-flow enzyme immunoconcentration for rapid detection of Listeria monocytogenes. Anal Bioanal Chem. 2013;405(10):3313–9.
Li Q, Zhang S, Cai Y, Yang Y, Hu F, Liu X, et al. Rapid detection of Listeria monocytogenes using fluorescence immunochromatographic assay combined with immunomagnetic separation technique. Int J Food Sci Technol. 2017;52(7):1559–66.
Hwang J, Lee S, Choo J. Application of a SERS-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin B. Nanoscale. 2016;8(22):11418–25.
Yeni F, Acar S, Polat OG, Soyer Y, Alpas H. Rapid and standardized methods for detection of foodborne pathogens and mycotoxins on fresh produce. Food Control. 2014;40:359–67.
Kim H-S, Cho I-H, Seo S-M, Jeon J-W, Paek S-H. In situ immuno-magnetic concentration-based biosensor systems for the rapid detection of Listeria monocytogenes. Mater Sci Eng C. 2012;32(2):160–6.
Kriz K, Gehrke J, Kriz D. Advancements toward magneto immunoassays. Biosens Bioelectron. 1998;13(7–8):817–23.
Hahm BK, Kim H, Singh AK, Bhunia AK. Pathogen enrichment device (PED) enables one-step growth, enrichment and separation of pathogen from food matrices for detection using bioanalytical platforms. J Microbiol Methods. 2015;117:64–73.
Kant K, Shahbazi M-A, Dave VP, Ngo TA, Chidambara VA, Than LQ, et al. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol Adv. 2018;36(4):1003–24.
Gehring AG, Albin DM, Bhunia AK, Kim H, Reed SA, Tu S-I. Mixed culture enrichment of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enterica, and Yersinia enterocolitica. Food Control. 2012;26(2):269–73.
Li F, Li F, Luo D, Lai W, Xiong Y, Xu H. Biotin-exposure-based immunomagnetic separation coupled with nucleic acid lateral flow biosensor for visibly detecting viable Listeria monocytogenes. Anal Chim Acta. 2018;1017:48–56.
Blažková M, Koets MH, Wichers J, van Amerongen A, Fukal L, Rauch P, Wichers JH, Vanamerongen A, Fukal L, Rauch P. Nucleic acid lateral flow immunoassay for the detection of pathogenic bacteria from food. Czech J Food Sci. 2009;27(SPEC. ISS.):S350–S353.
Wachiralurpan S, Sriyapai T, Areekit S, Kaewphinit T, Sriyapai P, Santiwatanakul S, et al. Development of a rapid screening test for Listeria monocytogenes in raw chicken meat using loop-mediated isothermal amplification (LAMP) and lateral flow dipstick (LFD). Food Anal Methods. 2017;10(11):3763–72.
Wang Y, Li H, Wang Y, Li H, Luo L, Xu J, et al. Development of multiple cross displacement amplification label-based gold nanoparticles lateral flow biosensor for detection of Listeria monocytogenes. Int J Nanomedicine. 2017;12:473–86.
Du XJ, Zang YX, Liu HB, Li P, Wang S. Recombinase polymerase amplification combined with lateral flow strip for Listeria monocytogenes detection in food. J Food Sci. 2018;83(4):1041–7.
Liu H-b, Du X-j, Zang Y-X, Li P, Wang S. SERS-based lateral flow strip biosensor for simultaneous detection of Listeria monocytogenes and Salmonella enterica serotype Enteritidis. J Agric Food Chem. 2017;65(47):10290–9.
Stambach NR, Carr SA, Cox CR, Voorhees KJ. Rapid detection of Listeria by bacteriophage amplification and SERS-lateral flow immunochromatography. Viruses. 2015;7(12):6631–41.
Austin JW, Pagotto FJ. Microbiology | detection of foodborne pathogens and their toxins. In: Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; 2003. p. 3886–92.
Baloch AR, Fučíková M, Rodina M, Metscher B, Tichopád T, Shah MA, et al. Delivery of iron oxide nanoparticles into primordial germ cells in sturgeon. Biomolecules. 2019;9(8):333.
Geng T, Kim KP, Gomez R, Sherman DM, Bashir R, Ladisch MR, et al. Expression of cellular antigens of Listeria monocytogenes that react with monoclonal antibodies C11E9 and EM-7G1 under acid-, salt- or temperature-induced stress environments. J Appl Microbiol. 2003;95(4):762–72.
Lathrop AA, Banada PP, Bhunia AK. Differential expression of InlB and ActA in Listeria monocytogenes in selective and nonselective enrichment broths. J Appl Microbiol. 2008;104(3):627–39.
Geng T, Hahm BK, Bhunia AK. Selective enrichment media affect the antibody-based detection of stress-exposed Listeria monocytogenes due to differential expression of antibody-reactive antigens identified by protein sequencing. J Food Prot. 2006;69(8):1879–86.
Jaradat ZW, Bhunia AK. Glucose and nutrient concentrations affect the expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl Environ Microbiol. 2002;68(10):4876–83.
Gilmartin N, Gião MS, Keevil CW, O’Kennedy R. Differential internalin A levels in biofilms of Listeria monocytogenes grown on different surfaces and nutrient conditions. Int J Food Microbiol. 2016;219:50–5.
Bai X, Liu D, Xu L, Tenguria S, Drolia R, Gallina NLF, et al. Biofilm-isolated Listeria monocytogenes exhibits reduced systemic dissemination at the early (12–24 h) stage of infection in a mouse model. Npj Biofilm Microbiome. 2021;7(1):18.
Knudsen GM, Fromberg A, Ng Y, Gram L. Sublethal concentrations of antibiotics cause shift to anaerobic metabolism in Listeria monocytogenes and induce phenotypes linked to antibiotic tolerance. Front Microbiol. 2016;7:1091.
Zhu X, Liu D, Singh AK, Drolia R, Bai X, Tenguria S, et al. Tunicamycin mediated inhibition of wall teichoic acid affect Staphylococcus aureus and Listeria monocytogenes cell morphology, biofilm formation and virulence. Front Microbiol. 2018;9:1352.
Rantsiou K, Greppi A, Garosi M, Acquadro A, Mataragas M, Cocolin L. Strain dependent expression of stress response and virulence genes of Listeria monocytogenes in meat juices as determined by microarray. Int J Food Microbiol. 2012;152(3):116–22.
Sokolovic Z, Schuller S, Bohne J, Baur A, Rdest U, Dickneite C, et al. Differences in virulence and in expression of PrfA and PrfA-regulated virulence genes of Listeria monocytogenes strains belonging to serogroup 4. Infect Immun. 1996;64(10):4008–19.
Marr A, Joseph B, Mertins S, Ecke R, Müller-Altrock S, Goebel W. Overexpression of PrfA leads to growth inhibition of Listeria monocytogenes in glucose-containing culture media by interfering with glucose uptake. J Bacteriol. 2006;188(11):3887–901.
Lathrop AA. Development of Listeria monocytogenes specific antibodies using a proteomics/genomics approach and expression of antibody-specific antigens InlB and ActA under different environments. West Lafayette: Purdue University; 2005.
Hahm BK, Bhunia AK. Effect of environmental stresses on antibody-based detection of Escherichia coli O157:H7, Salmonella enterica serotype Enteritidis and Listeria monocytogenes. J Appl Microbiol. 2006;100(5):1017–27.
Milohanic E, Glaser P, Coppee J-Y, Frangeul L, Vega Y, Vazquez-Boland JA, et al. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol Microbiol. 2003;47(6):1613–25.
Shetron-Rama LM, Mueller K, Bravo JM, Bouwer HA, Way SS, Freitag NE. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol Microbiol. 2003;48(6):1537–51.
Sue D, Fink D, Wiedmann M, Boor KJ. {sigma}B-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150(11):3843–55.
Lemes-Marques EG, Yano T. Influence of environmental conditions on the expression of virulence factors by Listeria monocytogenes and their use in species identification. FEMS Microbiol Lett. 2004;239(1):63–70.
Burkholder KM, Kim K-P, Mishra K, Medina S, Hahm B-K, Kim H, et al. Expression of LAP, a SecA2-dependent secretory protein, is induced under anaerobic environment. Microbes Infect. 2009;11(10–11):859–67.
Santiago NI, Zipf A, Bhunia AK. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl Environ Microbiol. 1999;65(6):2765–9.
Tasrip NA, Khairil Mokhtar NF, Hanapi UK, Abdul Manaf YN, Ali ME, Cheah YK, et al. Loop mediated isothermal amplification; a review on its application and strategy in animal species authentication of meat based food products. Int Food Res J. 2019;26(1):1–10.
Kim J, Easley CJ. Isothermal DNA amplification in bioanalysis: strategies and applications. Bioanalysis. 2011;3(2):227–39.
Li J, Macdonald J, Von Stetten F. Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. 2019;144(1):31–67.
Wang Y, Wang Y, Ma A-J, Li D-X, Luo L-J, Liu D-X, et al. Rapid and sensitive isothermal detection of nucleic-acid sequence by multiple cross displacement amplification. Sci Rep. 2015;5(1):1–16.
Ngom B, Guo Y, Wang X, Bi D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal Bioanal Chem. 2010;397(3):1113–35.
Roumani F, Azinheiro S, Carvalho J, Prado M, Garrido-Maestu A. Loop-mediated isothermal amplification combined with immunomagnetic separation and propidium monoazide for the specific detection of viable Listeria monocytogenes in milk products, with an internal amplification control. Food Control. 2021;125:107975.
Li J, Macdonald J, von Stetten F. A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. 2018;144(1):31–67.
Loessner MJ, Busse M. Bacteriophage typing of Listeria species. Appl Environ Microbiol. 1990;56(6):1912–8.
Schofield D, Sharp NJ, Westwater C. Phage-based platforms for the clinical detection of human bacterial pathogens. Bacteriophage. 2012;2(2):105–21.
Lindbäck T, Rottenberg ME, Roche SM, Rørvik LM. The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet Res. 2010;41(1):1–10.
Highmore CJ, Warner JC, Rothwell SD, Wilks SA, Keevil CW. Viable-but-nonculturable Listeria monocytogenes and Salmonella enterica serovar Thompson induced by chlorine stress remain infectious. mBio. 2018;9(2):e00540-00518.
Wideman NE, Oliver JD, Crandall PG, Jarvis NA. Detection and potential virulence of viable but non-culturable (VBNC) Listeria monocytogenes: a review. Microorganisms. 2021;9(1):194.
Kim H, Bhunia AK. SEL, a selective enrichment broth for simultaneous growth of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes. Appl Environ Microbiol. 2008;74(15):4853–66.
Sharma S, Zapatero-Rodríguez J, Estrela P, O'Kennedy R. Point-of-care diagnostics in low resource settings: present status and future role of microfluidics. Biosensors. 2015;5(3):577–601.
Kabanda T, Siedner MJ, Klausner JD, Muzoora C, Boulware DR. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis. 2014;58(1):113–6.
Petran RL, Swanson KMJ. Simultaneous growth of Listeria monocytogenes and Listeria innocua. J Food Prot. 1993;56(7):616–8.
Besse NG, Barre L, Buhariwalla C, Vignaud ML, Khamissi E, Decourseulles E, et al. The overgrowth of Listeria monocytogenes by other Listeria spp. in food samples undergoing enrichment cultivation has a nutritional basis. Int J Food Microbiol. 2010;136(3):345–51.
Corradini MG, Wang YL, Le A, Waxman SM, Zelent B, Chib R, et al. Identifying and selecting edible luminescent probes as sensors of food quality. AIMS Biophys. 2016;3(2):319.
Kim Y, Jang G, Lee TS. New fluorescent metal-ion detection using a paper-based sensor strip containing tethered rhodamine carbon nanodots. ACS Appl Mater Interfaces. 2015;7(28):15649–57.
Guo Z, Park S, Yoon J, Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev. 2014;43(1):16–29.
Swanson C, D'Andrea A. Lateral flow assay with near-infrared dye for multiplex detection. Clin Chem. 2013;59(4):641–8.
Alvarez MV, Moreira MR, Ponce A. Peroxidase activity and sensory quality of ready to cook mixed vegetables for soup: combined effect of biopreservatives and refrigerated storage. Food Sci Technol. 2015;35(1):86–94.
Jiang Y, Chen S, Zhao Y, Yang X, Fu S, McKillip JL, et al. Multiplex loop-mediated isothermal amplification-based lateral flow dipstick for simultaneous detection of 3 food-borne pathogens in powdered infant formula. J Dairy Sci. 2020;103(5):4002–12.
Wang Y, Yan W, Fu S, Hu S, Wang Y, Xu J, et al. Multiple cross displacement amplification coupled with nanoparticles-based lateral flow biosensor for detection of Staphylococcus aureus and identification of methicillin-resistant S. aureus. Front Microbiol. 2018;9:907.
Andryukov BG. Six decades of lateral flow immunoassay: from determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020;6(3):280.
Funding
The work in the authors’ laboratories was supported by funds from the Brazilian Ministry of Health for the Improvement of Higher Education Personnel (CAPES grant # 88882.385457/2007-01 2019-2023 and grant # 88887.608185/2021-00 2021-2025) and by the US Department of Agriculture, Agricultural Research Service, under Agreement No. 59-8072-6-001. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fogaça, M.B.T., Bhunia, A.K., Lopes-Luz, L. et al. Antibody- and nucleic acid–based lateral flow immunoassay for Listeria monocytogenes detection. Anal Bioanal Chem 413, 4161–4180 (2021). https://doi.org/10.1007/s00216-021-03402-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03402-8