Abstract
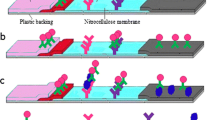
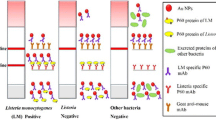
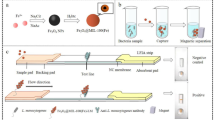
Lateral-flow enzyme immunochromatography coupled with an immunomagnetic step was developed for rapid detection of Listeria monocytogenes in food matrices. The target bacteria was first separated and concentrated by magnetic nanoparticles containing the enzyme and directly applied to the assay system to induce an antigen–antibody reaction without any additional steps. The color signals produced by an enzyme–substrate reaction at a specific site on the immunostrip were found to be directly proportional to the concentration of L. monocytogenes in the sample. The detection concept was demonstrated by performing an enzyme immunoassay on a microtiter well prior to applying it to the lateral-flow assay. Results of the chromatographic analysis yield a limit of detection of 95 and 97 ± 19.5 CFU/mL in buffer solution and 2 % milk sample, respectively. In addition to the high sensitivity, it was also possible to shorten the separation and detection time to within 2 h. The system also showed no cross-reactivity with other bacteria (e.g., Escherichia coli O157:H7, Salmonella typhimurium, and Salmonella enteritidis). The analytical procedure developed will enable us to not only utilize the assay in the field where fast screening for pathogenic agents is required but also as a preventive measure to contain disease outbreak.





Similar content being viewed by others
References
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM (2011) Emerg Infect Dis 17:7–15
Shyamal KM, Ronald N, Frederick CP (1986) Appl Environ Microbiol 52:510–514
Schelch WF, Acheson D (2000) Clin Infect Dis 31:770–775
Dykes GA, Dworaczek M (2002) Lett Appl Microbiol 35:538–542
Velusamy V, Arshak K, Korostynska O, Oliwa K, Adley C (2010) Biotechnol Adv 28:232–254
Scotter SL, Langton S, Lombard B, Schulten S, Nagelkerke N, Veld PH, Rollier P, Lahellec C (2001) Int J Food Microbiol 64:295–306
Reissbrodt R (2004) Int J Food Microbiol 95:1–9
Sütterlin K, Englert R, Schmidt-Wieland T, Schmitt J, Reischl U, Lehn NJ (2003) Clin Microbiol 41:3449
Navas J, Ortiz S, Lopez P, Jantzen MM, Lopez V, Martinez JV (2006) Foodborne Pathog Dis 3:347–354
Ueda S, Maruyama T, Kuwabara Y (2006) Biocontrol Sci 11:129–134
Xiulan S, Xiaolian Z, Jian T, Zhou J, Chu FS (2005) Int J Food Microbiol 99:185–194
Wang J, Wang X, Li Y, Yan S, Zhou Q, Gao B, Peng J, Du J, Fu Q, Jia S, Zhang J, Zhan L (2012) Anal Sci 28:237–241
Seo SM, Cho IH, Kim JH, Jeon JW, Oh EK, Yu HS, Shin SB, Lee HJ, Paek SH (2009) Bull Korean Chem Soc 30:2993–2998
Muldoon MT, Allen AC, Gonzalez V, Sutzko M, Klaus L (2012) J AOAC Int 95:850–859
Alles S, Curry S, Almy D, Jagadeesan B, Rice J, Mozola M (2012) J AOAC Int 95:424–434
Kitao T, Miyoshi-Akiyama T, Shimada K, Tanaka M, Narahara K, Saito N, Kirikae TJ (2010) Antimicrob Chemother 65:1382–1386
Noguera P, Posthuma-Trumpie GA, Van Tuil M, Van der Wal FJ, De Boer A, Moer APHA, Van Amerongen A (2011) Anal Bioanal Chem 399:831–838
Galikowska E, Kunikowska D, Tokarska-Pietrzak E, Dziadziuszko H, Łoś JM, Golec P, Węgrzyn G, Łoś M (2011) Eur J Clin Microbiol Infect Dis 30:1067–1073
Johnson RP, Durham RJ, Johnson ST, Macdonald LA, Jeffrey SR, Butman BT (1995) Appl Environ Microbiol 61:386–388
Cho JH, Paek EH, Cho IH, Paek SH (2005) Anal Chem 77:4091–4097
Seo SM, Cho IH, Jeon JW, Cho HK, Oh EK, Yu HS, Shin SB, Lee HJ, Paek SH (2010) J Food Protect 73:1466–1473
Wang Y, Ravindranath S, Irudayaraj J (2011) Anal Bioanal Chem 399:1271–1278
Ravindranath S, Wang Y, Irudayaraj J (2011) Sens Actuators B Chem 152:183–190
Cho JH, Han SM, Paek EH, Cho IH, Paek SH (2006) Anal Chem 78:793–800
Binder R, Archimbaud Y (2000) Regul Toxicol Pharm 31:S23–S26
Abadias M, Usall J, Anguera M, Solson C, Vinas I (2008) Int J Food Microbiol 123:121–129
Atanassova V, Reich F, Klein G (2008) J Food Prot 71:860–864
Esteban JI, Oporto B, Aduriz G, Juste RA, Hurtado A (2008) Int J Food Microbiol 123:177–182
Murphy NM, McLauchlin J, Ohai C, Grant KA (2007) Int J Food Microbiol 120:110–119
Hoffman AD, Wiedmann M (2001) J Food Prot 64:1521–1526
Shearer AEH, Strapp CM, Joerger RD (2001) J Food Prot 64:788–795
Heather S, Helene M (2006) Infect Immun 74:6675–6681
West HG (2008) Appetite 51:25–29
Gupta M, Irudayaraj J, Schmilovitch Z, Mizrach A (2006) Trans ASABE 49:1249–1256
Yu C, Ganjoo A, Jain H, Pantano C, Irudayaraj J (2005) Anal Chem 78:2500–2506
Acknowledgment
This work was supported by funding from the Center for Food Safety Engineering through a collaborative grant between USDA-ARS and Purdue University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, IH., Irudayaraj, J. Lateral-flow enzyme immunoconcentration for rapid detection of Listeria monocytogenes . Anal Bioanal Chem 405, 3313–3319 (2013). https://doi.org/10.1007/s00216-013-6742-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6742-3