Abstract
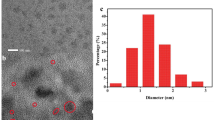
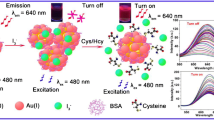
In this work, a facile, label-free, and sensitive fluorometric strategy for detection of trypsin and its inhibitor was established on the basis of the fluorescence resonance energy transfer (FRET) between mercaptoundecanoic acid functionalized gold nanoclusters (AuNCs) and gold nanoparticles (AuNPs) via protamine as a bridge. Protamine can trigger the aggregation of AuNPs and link AuNCs with aggregated AuNPs through electrostatic interaction. Compared with monodisperse AuNPs, the UV–vis absorption band of aggregated AuNPs overlapped considerably with the emission spectrum of AuNCs. Thus, the fluorescence of AuNCs was obviously quenched by the aggregated AuNPs through FRET. In the presence of trypsin, protamine was hydrolyzed into small fragments, leading to the deaggregation of AuNPs and breaking of the short distance between AuNPs and AuNCs, so the FRET process was inhibited, and the fluorescence of AuNCs was recovered. The increase in the fluorescence intensity of AuNCs was directly related to the amount of trypsin. Hence trypsin can be determined on the basis of the variation of fluorescence intensity, with a linear range of 5–5000 ng mL-1 and a detection limit of 1.9 ng mL-1. In addition, this system was used for the detection of trypsin inhibitor by application of the inhibitor isolated from soybean as a model. The sensing method was applied for trypsin detection in human urine and commercial multienzyme tablet samples with satisfactory results.

ᅟ






Similar content being viewed by others
References
Cleemann F, Karuso P. Fluorescence anisotropy assay for the traceless kinetic analysis of protein digestion. Anal Chem. 2008;80(11):4170–4.
Alfonta L, Blumenzweig I, Zayats M, Baraz L, Kotler M, Willner I. Electronic transduction of HIV-1 drug resistance in AIDS patients. Chembiochem. 2004;5(7):949–57.
Griffiths SW, Cooney CL. Relationship between protein structure and methionine oxidation in recombinant human α1-antitrypsin. Biochemistry. 2002;41(20):6245–52.
Lopez-Otin C, Overall CM. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3(7):509–19.
Olsen JV, Ong SE, Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol Cell Proteomics. 2004;3(6):608–14.
Hirota M, Ohmuraya M, Baba H. The role of trypsin, trypsin inhibitor, and trypsin receptor in the onset and aggravation of pancreatitis. J Gastroenterol. 2006;41(9):832–6.
Artigas JM, Garcia ME, Faure MR, Gimeno AM. Serum trypsin levels in acute pancreatic and non-pancreatic abdominal conditions. Postgrad Med J. 1981;57(666):219–22.
Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84(2):579–621.
Wilcken B, Brown AR, Urwin R, Brown DA. Cystic fibrosis screening by dried blood spot trypsin assay: results in 75,000 newborn infants. J Pediatr. 1983;102(3):383–7.
Goldberg DM. Proteases in the evaluation of pancreatic function and pancreatic disease. Clin Chim Acta. 2000;291(2):201–21.
Miao P, Liu T, Li X, Ning L, Yin J, Han K. Highly sensitive, label-free colorimetric assay of trypsin using silver nanoparticles. Biosens Bioelectron. 2013;49:20–4.
Chen L, Fu X, Li J. Ultrasensitive surface-enhanced Raman scattering detection of trypsin based on anti-aggregation of 4-mercaptopyridine-functionalized silver nanoparticles: an optical sensing platform toward proteases. Nanoscale. 2013;5(13):5905–11.
Dai H, Chen S, Li Y, Zeng B, Zhang S, Hong Z, et al. Photoelectrochemical biosensor constructed using TiO2 mesocrystals based multipurpose matrix for trypsin detection. Biosens Bioelectron. 2017;92:687–94.
Poon CY, Li QH, Zhang JL, Li ZP, Dong C, Lee AWM, et al. FRET-based modified graphene quantum dots for direct trypsin quantification in urine. Anal Chim Acta. 2016;917:64–70.
Lu YZ, Chen W. Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chem Soc Rev. 2012;41(9):3594–623.
Shang L, Dong S, Nienhaus GU. Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today. 2011;6(4):401–18.
Wang M, Lin Z, Liu Q, Jiang S, Liu H, Su X. DNA-hosted copper nanoclusters/graphene oxide based fluorescent biosensor for protein kinase activity detection. Anal Chim Acta. 2018;1012:66–73.
Wang M, Wang S, Su D, Su X. Copper nanoclusters/polydopamine nanospheres based fluorescence aptasensor for protein kinase activity determination. Anal Chim Acta. 2018; https://doi.org/10.1016/j.aca.2018.06.043.
Sun J, Jin Y. Fluorescent Au nanoclusters: recent progress and sensing applications. J Mater Chem C. 2014;2(38):8000–11.
Yuan X, Zhang B, Luo Z, Yao Q, Leong DT, Yan N, et al. Balancing the rate of cluster growth and etching for gram-scale synthesis of thiolate-protected Au25 nanoclusters with atomic precision. Angew Chem Int Ed. 2014;53(18):4623–7.
Chang HC, Chang YF, Fan NC, Ho JAA. Facile preparation of high-quantum-yield gold nanoclusters: application to probing mercuric ions and biothiols. ACS Appl Mater Interfaces. 2014;6(21):18824–31.
Ban R, Abdel-Halim ES, Zhang J, Zhu J-J. β-Cyclodextrin functionalised gold nanoclusters as luminescence probes for the ultrasensitive detection of dopamine. Analyst. 2015;140(4):1046–53.
Deng H-H, Wang F-F, Shi X-Q, Peng H-P, Liu A-L, Xia X-H, et al. Water-soluble gold nanoclusters prepared by protein-ligand interaction as fluorescent probe for real-time assay of pyrophosphatase activity. Biosens Bioelectron. 2016;83:1–8.
Sun J, Yang F, Zhao D, Yang X. Highly sensitive real-time assay of inorganic pyrophosphatase activity based on the fluorescent gold nanoclusters. Anal Chem. 2014;86(15):7883–9.
Grabar KC, Brown KR, Keating CD, Stranick SJ, Tang SL, Natan MJ. Nanoscale characterization of gold colloid monolayers: a comparison of four techniques. Anal Chem. 1997;69(3):471–7.
Zhao W, Brook MA, Li Y. Design of gold nanoparticle-based colorimetric biosensing assays. Chembiochem. 2008;9(15):2363–71.
Sun J, Zhang J, Jin Y. 11-Mercaptoundecanoic acid directed one-pot synthesis of water-soluble fluorescent gold nanoclusters and their use as probes for sensitive and selective detection of Cr3+ and Cr6+. J Mater Chem C. 2013;1(1):138–43.
Fu XL, Chen LX, Li JH. Ultrasensitive colorimetric detection of heparin based on self-assembly of gold nanoparticles on graphene oxide. Analyst. 2012;137(16):3653–8.
Jena BK, Raj CR. Optical sensing of biomedically important polyionic drugs using nano-sized gold particles. Biosens Bioelectron. 2008;23(8):1285–90.
Sun J, Yang XR. Gold nanoclusters-Cu2+ ensemble-based fluorescence turn-on and real-time assay for acetylcholinesterase activity and inhibitor screening. Biosens Bioelectron. 2015;74:177–82.
Liu X, Li Y, Jia L, Chen S, Shen Y. Ultrasensitive fluorescent detection of trypsin on the basis of surfactant-protamine assembly with tunable emission wavelength. RSC Adv. 2016;6(96):93551–7.
Lin YY, Chapman R, Stevens MM. Label-free multimodal protease detection based on protein/perylene dye coassembly and enzyme-triggered disassembly. Anal Chem. 2014;86(13):6410–7.
Chen X, Wang J, Yang C, Ge Z, Yang H. Fluorescence resonance energy transfer from NaYF4:Yb,Er to nano gold and its application for glucose determination. Sensors Actuators B Chem. 2018;255:1316–24.
Wu X, Song Y, Yan X, Zhu C, Ma Y, Du D, et al. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens Bioelectron. 2017;94:292–7.
Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat Rev Drug Discov. 2010;9(9):690–701.
Wang M, Wang L, Liu Q, Su X. A fluorescence sensor for protein kinase activity detection based on gold nanoparticles/copper nanoclusters system. Sensors Actuators B. 2018;256:691–8.
Zhang QF, Li WY, Chen J, Wang FY, Wang Y, Chen Y, et al. An ultrasensitive chemiluminescence turn-on assay for protease and inhibitor screening with a natural substrate. Chem Commun. 2013;49(30):3137–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21775052 and no. 21575048) and the Science and Technology Development Project of Jilin Province, China (no. 20180414013GH).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical committee approval
All experiments were performed in compliance with the relevant laws and institutional guidelines, and were approved by the Ethics Committee of Changchun China Japan Union Hospital. Written informed consent for all samples was obtained from the human participants.
Human and animal rights
There was no violation of human or animal rights during this work.
Electronic supplementary material
ESM 1
(PDF 534 kb)
Rights and permissions
About this article
Cite this article
Wang, M., Su, D., Wang, G. et al. A fluorometric sensing method for sensitive detection of trypsin and its inhibitor based on gold nanoclusters and gold nanoparticles. Anal Bioanal Chem 410, 6891–6900 (2018). https://doi.org/10.1007/s00216-018-1292-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1292-3