Abstract
A new technology for rapid, automated coupling of solid-phase microextraction and mass spectrometry is introduced. Use of a so-called capillary gap sampler for automated solid-phase microextraction and direct delivery of the extracted analytes to a mass spectrometer provides certain advantages over existing technologies: coupling of the capillary gap sampler to a mass spectrometer offers quick, automated, and site-specific extraction from very low volume samples. High stability, reusability, and repeatability were achieved through systematic optimization. Diazepam, oxazepam, and nordiazepam were used as test compounds in all experiments. The ability of the sampler to extract benzodiazepines from human plasma (limit of detection 0.3 μg/mL) in the therapeutic range was confirmed. A linear dynamic range from 1 to 1000 ng/mL for all three analytes was found. The relative standard deviation of 20 extractions was between 11% and 17%, for oxazepam, nordiazepam, and diazepam, indicating acceptable repeatability of the method.

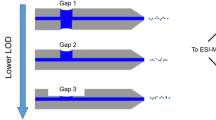
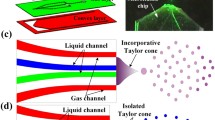
Schematic of the desorption step inside the liquid bridge formed between two capillaries in thecapillary gap sampler








Similar content being viewed by others
References
Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem. 1990;62:2145–8. https://doi.org/10.1021/ac00218a019.
Zhang Z, Yang MJ, Pawliszyn J. Solid-phase microextraction. A solvent-free alternative for sample preparation. Anal Chem. 1994;66:844A–53A. https://doi.org/10.1021/ac00089a001.
Theodoridis G, Koster EH, de Jong GJ. Solid-phase microextraction for the analysis of biological samples. J Chromatogr B. 2000;745:49–82.
Ulrich S. Solid-phase microextraction in biomedical analysis. J Chromatogr A. 2000;902:167–94. https://doi.org/10.1016/S0021-9673(00)00934-1.
Snow NH. Solid-phase micro-extraction of drugs from biological matrices. J Chromatogr A. 2000;885:445–55.
Sides SL, Polowy KB, Thornquest AD Jr, Burinsky DJ. Identification of a pharmaceutical packaging off-odor using solid phase microextraction gas chromatography/mass spectrometry. J Pharm Biomed Anal. 2001;25:379–86. https://doi.org/10.1016/S0731-7085(00)00517-3.
Teitz DS, Khan S, Powell ML, Jemal M. An automated method of sample preparation of biofluids using pierceable caps to eliminate the uncapping of the sample tubes during sample transfer. J Biochem Biophys Methods. 2000;45:193–204.
Jemal M, Teitz D, Ouyang Z, Khan S. Comparison of plasma sample purification by manual liquid-liquid extraction, automated 96-well liquid-liquid extraction and automated 96-well solid-phase extraction for analysis by high-performance liquid chromatography with tandem mass spectrometry. J Chromatogr B. 1999;732:501–8.
Mitani K, Fujioka M, Kataoka H. Fully automated analysis of estrogens in environmental waters by in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2005;1081:218–24. https://doi.org/10.1016/j.chroma.2005.05.058.
Tong XS, Wang J, Zheng S, Pivnichny JV. High-throughput pharmacokinetics screen of VLA-4 antagonists by LC/MS/MS coupled with automated solid-phase extraction sample preparation. J Pharm Biomed Anal. 2004;35:867–77. https://doi.org/10.1016/j.jpba.2004.02.017.
Lord HL, Grant RP, Walles M, Incledon B, Fahie B, Pawliszyn JB. Development and evaluation of a solid-phase microextraction probe for in vivo pharmacokinetic studies. Anal Chem. 2003;75:5103–15. https://doi.org/10.1021/ac0343230.
Rule G, Chapple M, Henion J. A 384-well solid-phase extraction for LC/MS/MS determination of methotrexate and its 7-hydroxy metabolite in human urine and plasma. Anal Chem. 2001;73:439–43. https://doi.org/10.1021/ac000897i.
Rule G, Henion J. High-throughput sample preparation and analysis using 96-well membrane solid-phase extraction and liquid chromatography-tandem mass spectrometry for the determination of steroids in human urine. J Am Soc Mass Spectrom. 1999;10:1322–7. https://doi.org/10.1016/S1044-0305(99)00107-5.
Kaye B, Herron WJ, Macrae PV, Robinson S, Stopher DA, Venn RF, et al. Rapid, solid phase extraction technique for the high-throughput assay of darifenacin in human plasma. Anal Chem. 1996;68:1658–60. https://doi.org/10.1021/ac9507552.
Hutchinson JP, Setkova L, Pawliszyn J. Automation of solid-phase microextraction on a 96-well plate format. J ChromatogrA. 2007;1149:127–37. https://doi.org/10.1016/j.chroma.2007.02.117.
Roddy TP, Horvath CR, Stout SJ, Kenney KL, Ho P-I, Zhang J-H, et al. Mass spectrometric techniques for label-free high-throughput screening in drug discovery. Anal Chem. 2007;79:8207–13. https://doi.org/10.1021/ac062421q.
Biddlecombe RA, Benevides C, Pleasance S. A clinical trial on a plate? The potential of 384-well format solid phase extraction for high-throughput bioanalysis using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:33–40. https://doi.org/10.1002/1097-0231(20010115)15:1<33::AID-RCM188>3.0.CO;2-4.
Vatinno R, Vuckovic D, Zambonin CG, Pawliszyn J. Automated high-throughput method using solid-phase microextraction-liquid chromatography-tandem mass spectrometry for the determination of ochratoxin A in human urine. J Chromatogr A. 2008;1201:215–21. https://doi.org/10.1016/j.chroma.2008.05.079.
Cudjoe E, Vuckovic D, Hein D, Pawliszyn J. Investigation of the effect of the extraction phase geometry on the performance of automated solid-phase microextraction. Anal Chem. 2009;81:4226–32. https://doi.org/10.1021/ac802524w.
Cudjoe E, Pawliszyn J. A new approach to the application of solid phase extraction disks with LC–MS/MS for the analysis of drugs on a 96-well plate format. J Pharm Biomed Anal. 2009;50:556–62. https://doi.org/10.1016/j.jpba.2008.07.014.
Vuckovic D, Cudjoe E, Hein D, Pawliszyn J. Automation of solid-phase microextraction in high-throughput format and applications to drug analysis. Anal Chem. 2008;80:6870–80. https://doi.org/10.1021/ac800936r.
Deng J, Yang Y, Wang X, Luan T. Strategies for coupling solid-phase microextraction with mass spectrometry. Trends Anal Chem. 2014;55:55–67. https://doi.org/10.1016/j.trac.2013.12.004.
Marsili RT. SPME−MS−MVA as an electronic nose for the study of off-flavors in milk. J Agric Food Chem. 1999;47:648–54. https://doi.org/10.1021/jf9807925.
Pérès C, Viallon C, Berdagué J-L. Solid-phase microextraction-mass spectrometry: a new approach to the rapid characterization of cheeses. Anal Chem. 2001;73:1030–6. https://doi.org/10.1021/ac001146j.
Möder M, Löster H, Herzschuh R, Popp P. Determination of urinary acylcarnitines by ESI-MS coupled with solid-phase microextraction (SPME). J Mass Spectrom. 1997;32:1195–204. https://doi.org/10.1002/(SICI)1096-9888(199711)32:11<1195::AID-JMS578>3.0.CO;2-9.
Chen J, Pawliszyn J. Solid phase microextraction coupled to high-performance liquid chromatography. Anal Chem. 1995;67:2530–2533. https://doi.org/10.1021/ac00111a006.
Ceglarek U, Efer J, Schreiber A, Zwanziger E, Engewald W. Determination of linear alkylbenzenesulfonates in communal wastewater by means of solid phase microextraction coupled with API-MS and HPLC-FLD. Fresenius J Anal Chem. 1999;365:674–81. https://doi.org/10.1007/s002160051544.
McCooeye MA, Mester Z, Ells B, Barnett DA, Purves RW, Guevremont R. Quantitation of amphetamine, methamphetamine, and their methylenedioxy derivatives in urine by solid-phase microextraction coupled with electrospray ionization−high-field asymmetric waveform ion mobility spectrometry−mass spectrometry. Anal Chem. 2002;74:3071–5. https://doi.org/10.1021/ac011296.
van Hout MWJ, Jas V, Niederländer HAG, de Zeeuw RA, de Jong GJ. Non-equilibrium solid-phase microextraction coupled directly to ion-trap mass spectrometry for rapid analysis of biological samples. Analyst. 2002;127:355–9. https://doi.org/10.1039/B110729A.
van Hout MWJ, Jas V, Niederländer HAG, de Zeeuw RA, de Jong GJ. Ultra-rapid non-equilibrium solid-phase microextraction at elevated temperatures and direct coupling to mass spectrometry for the analysis of lidocaine in urine. J Sep Sci. 2003;26:1563–8. https://doi.org/10.1002/jssc.200301567.
Mirabelli MF, Wolf J-C, Zenobi R. Direct coupling of solid-phase microextraction with mass spectrometry: sub-pg/g sensitivity achieved using a dielectric barrier discharge ionization source. Anal Chem. 2016;88:7252–8. https://doi.org/10.1021/acs.analchem.6b01507.
Neu V, Steiner R, Müller S, Fattinger C, Zenobi R. Development and characterization of a capillary gap sampler as new microfluidic device for fast and direct analysis of low sample amounts by ESI-MS. Anal Chem. 2013;85:4628–35. https://doi.org/10.1021/ac400186t.
Neu V, Dörig P, Fattinger C, Müller S, Zenobi R. Characterization of a miniaturized liquid bridge for nL sample infusion: a comparative study of sample flush-out behavior using flow simulations and direct ESI-MS analysis. Microfluid Nanofluid. 2016;20:62. https://doi.org/10.1007/s10404-016-1732-3.
Mirnaghi FS, Chen Y, Sidisky LM, Pawliszyn J. Optimization of the coating procedure for a high-throughput 96-blade solid phase microextraction system coupled with LC–MS/MS for analysis of complex samples. Anal Chem. 2011;83:6018–25. https://doi.org/10.1021/ac2010185.
Pawliszyn J, editor. Handbook of solid phase microextraction. Amsterdam: Elsevier; 2011.
Lavaud S, Canivet E, Wuillai A, Maheut H, Randoux C, Bonnet J-M, et al. Optimal anticoagulation strategy in haemodialysis with heparin-coated polyacrylonitrile membrane. Nephrol Dial Transplant. 2003;18:2097–104. https://doi.org/10.1093/ndt/gfg272.
Nie F-Q, Xu Z-K, Ming Y-Q, Kou R-Q, Liu Z-M, Wang S-Y. Preparation and characterization of polyacrylonitrile-based membranes: effects of internal coagulant on poly(acrylonitrile-co-malefic acid) ultrafiltration hollow fiber membranes. Desalination. 2004;160:43–50. https://doi.org/10.1016/S0011-9164(04)90016-1.
Mirnaghi FS, Pawliszyn J. Reusable solid-phase microextraction coating for direct immersion whole-blood analysis and extracted blood spot sampling coupled with liquid chromatography–tandem mass spectrometry and direct analysis in real time–tandem mass spectrometry. Anal Chem. 2012;84:8301–9. https://doi.org/10.1021/ac3018229.
Mullett WM, Pawliszyn J. The development of selective and biocompatible coatings for solid phase microextraction. J Sep Sci. 2003;26:251–60. https://doi.org/10.1002/jssc.200390031.
Alam MN, Ricardez-Sandoval L, Pawliszyn J. Numerical modeling of solid-phase microextraction: binding matrix effect on equilibrium time. Anal Chem. 2015;87:9846–54. https://doi.org/10.1021/acs.analchem.5b02239.
Pawliszyn J. Sample preparation: quo vadis? Anal Chem. 2003;75:2543–58. https://doi.org/10.1021/ac034094h.
Mulder J. Basic principles of membrane technology. Dordrecht: Kluwer; 1996.
Zhang Z, Poerschmann J, Pawliszyn J. Direct solid phase microextraction of complex aqueous samples with hollow fibre membrane protection. Anal Commun. 1996;33:219–21. https://doi.org/10.1039/AC9963300219.
Rasmussen KE, Pedersen-Bjergaard S, Krogh M, Grefslie Ugland H, Grønhaug T. Development of a simple in-vial liquid-phase microextraction device for drug analysis compatible with capillary gas chromatography, capillary electrophoresis and high-performance liquid chromatography. J Chromatogr A. 2000;873:3–11. https://doi.org/10.1016/S0021-9673(99)01163-2.
Boos K-S, Grimm C-H. High-performance liquid chromatography integrated solid-phase extraction in bioanalysis using restricted access precolumn packings. Trends Anal Chem. 1999;18:175–80. https://doi.org/10.1016/S0165-9936(98)00102-2.
Stead AH, Moffat AC. A collection of therapeutic, toxic and fatal blood drug concentrations in man. Hum Toxicol. 1983;2:437–64.
Brown S. Clarke’s isolation and identification of drugs. J Clin Pathol. 1986;39:1368.
Winek CL, Wahba WW, Winek CL, Balzer TW. Drug and chemical blood-level data 2001. Forensic Sci Int. 2001;122:107–23.
Schulz M, Schmoldt A. Therapeutic and toxic blood concentrations of more than 500 drugs. Pharmazie. 1997;52:895–911.
Repetto MR, Repetto M. Habitual, toxic, and lethal, and lethal concentration of 103 drugs of abuse in humans. J Toxicol. 1997;35:1–9. https://doi.org/10.3109/15563659709001158.
Jones AW, Holmgren A, Holmgren P. High concentrations of diazepam and nordiazepam in blood of impaired drivers: association with age, gender and spectrum of other drugs present. Forensic Sci Int. 2004;146:1–7. https://doi.org/10.1016/j.forsciint.2004.05.020.
Acknowledgements
We gratefully thank Christof Fattinger for his support of sampler development and during this project and Pablo Dörig for simulations of the flow profile with Comsol Multiphysics. Moreover, we thank the Scientific Center for Optical and Electron Microscopy of ETH Zurich for providing us with resources and services in electron microscopy. We thank the Swiss National Science Foundation for financial support of this project (grant no. 200020_159929). YZ was funded by a Pao Yu-Kong and Pao Zhao-Long scholarship for Chinese scientists studying abroad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 1.03 mb)
Rights and permissions
About this article
Cite this article
Ghiasikhou, S., da Silva, M.F., Zhu, Y. et al. The capillary gap sampler, a new microfluidic platform for direct coupling of automated solid-phase microextraction with ESI-MS. Anal Bioanal Chem 409, 6873–6883 (2017). https://doi.org/10.1007/s00216-017-0652-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0652-8