Abstract
In this study, we present a novel design of interference-free, negligible installation-induced stress, suitable for the fabrication of high-throughput quartz crystal microbalance (HQCM) chips. This novel HQCM chip configuration was fabricated using eight independent yet same-batch quartz crystal resonators within a common glass substrate with eight through-holes of diameter slightly larger than that of the quartz resonator. Each quartz resonator’s rim was adhered to the inner part of the through-hole via silicone glue to form the rigid (quartz)-soft (silicone)-rigid (glass) structure (RSRS) which effectively eliminates the acoustic couplings among different resonators and largely alleviates the installation-induced stresses. The consistence of the eight resonators was verified by very similar equivalent circuit parameters and very close response slopes to liquid density and viscosity. The HQCM chip was then employed for real-time and continuous monitoring of H9C2 cardiomyoblast adhesions and viscoelastic changes induced by the treatments of two types of drugs: drugs that affect the cytoskeletons, including nocodazole, paclitaxel, and Y-27632, and drugs that affect the contractile properties of the cells: verapamil and different dosages of isoprenaline. Meanwhile, we compared the cytoskeleton affecting drug-induced viscoelastic changes of H9C2 with those of human umbilical vein endothelial cells (HUVECs). The results described here provide the first solution to fabricate HQCM chips that are free from the limitation of resonator number, installation-induced stress, and acoustic interferences among resonators, which should find wide applications in areas of cell phenotype assay, cytotoxicity test, drug evaluation and screening, etc.

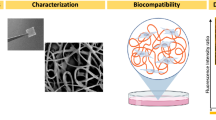
Schematic illustration of the principle and configuration of interference-free high-throughput QCM chip to evaluate and screen drugs based on cell viscoelasticity.






Similar content being viewed by others
References
Arnau AA. Review of interface electronic systems for AT-cut quartz crystal microbalance applications in liquids. Sensors. 2008;8:370–411.
Pavey KD. Quartz crystal analytical sensors: the future of label-free, real-time diagnostics? Expert Rev Mol Diagn. 2002;2:173–86.
Kanazawa KK, Gordon JG. The oscillation frequency of a quartz resonator in contact with liquid. Anal Chim Acta. 1985;175:99–105.
Marx KA, Zhou T, Montrone A, Schulze H, Braunhut SJ. A quartz crystal microbalance cell biosensor: detection of microtubule alterations in living cells at nM nocodazole concentrations. Biosens Bioelectron. 2001;16:773–82.
Haider L, Gindre M, Guillou-Buffelo DL, Laugier P, Perrot H, Carreiras F, et al. Cell attachment and spreading processes monitored by the thickness shear-mode quartz sensor. IEEE Sensors J. 2004;4:535–42.
Dan S, Lange S, Kholia S, Jorfi S, Antwi-Baffour S, Inal J. Label-free real-time acoustic sensing of microvesicle release from prostate cancer (PC3) cells using a quartz crystal microbalance. Biochem Biophys Res Commun. 2014;453:619–24.
Zhou T, Marx KA, Warren M, Schulze H, Braunhut SJ. The quartz crystal microbalance as a continuous monitoring tool for the study of endothelial cell surface attachment and growth. Biotechnol Prog. 2000;16:268–77.
Hirst ER, Yuan YJ, Xu WL, Bronlund JE. Bond-rupture immunosensors—a review. Biosens Bioelectron. 2008;23:1759–68.
Kim N, Park IS, Kim DK. High-sensitivity detection for model organophosphorus and carbamate pesticide with quartz crystal microbalance-precipitation sensor. Biosens Bioelectron. 2007;22:1593–9.
Marx KA, Zhou T, Warren M, Braunhut SJ. Quartz crystal microbalance study of endothelial cell number dependent differences in initial adhesion and steady-state behavior: evidence for cell-cell cooperativity in initial adhesion and spreading. Biotechnol Prog. 2003;19:987–99.
Xi J, Chen J, Garcia M, Penn L. Quartz crystal microbalance in cell biology studies. J Biochip Tissue Chips. 2013;5:2153–0777.
Xi B, Yu N, Wang X, Xu X, Abassi Y. The application of cell-based label-free technology in drug discovery. Biotechnol J. 2008;3:484–95.
Tuantranont A, Wisitsoraat A, Sritongkham P, Jaruwongrungsee K. A review of monolithic multichannel quartz crystal microbalance: a review. Anal Chim Acta. 2011;687:114–28.
Jaruwongrungsee K, Waiwijit U, Wisitsoraat A, Sangworasil M, Pintavirooj C, Tuantranont A. Real-time multianalyte biosensors based on interference-free multichannel monolithic quartz crystal microbalance. Biosens Bioelectron. 2014;67:576–81.
Suresh S. Biomechanics and biophysics of cancer cells. Acta Mater. 2007;55:3989–4014.
Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–3.
Wu S, Liu X, Zhou X, Liang XM, Gao D, Liu H, et al. Quantification of cell viability and rapid screening anti-cancer drug utilizing nanomechanical fluctuation. Biosens Bioelectron. 2016;77:164–73.
Hug TS. Biophysical methods for monitoring cell-substrate interactions in drug discovery. Assay Drug Dev Technol. 2003;1:479–88.
Xu Y, Lv Y, Wang L, Xing W, Cheng J. A microfluidic device with passive air-bubble valves for real-time measurement of dose-dependent drug cytotoxicity through impedance sensing. Biosens Bioelectron. 2012;32:300–4.
Hong J, Kandasamy K, Marimuthu M, Choi CS, Kim S. Electrical cell-substrate impedance sensing as a non-invasive tool for cancer cell study. Analyst. 2011;136:237–45.
Orgovan N, Kovacs B, Farkas E, Szabó B, Zaytseva N, Fang Y, et al. Bulk and surface sensitivity of a resonant waveguide grating imager. Appl Phys Lett. 2014;104:083506.
Zhou T, Zhou Z, Zhou S, Huang F. Real-time monitoring of contractile properties of H9C2 cardiomyoblasts by using a quartz crystal microbalance. Anal Methods-UK. 2016;8:488–95.
Kunze A, Steel D, Dahlenborg K, Sartipy P, Svedhem S. Non-invasive acoustical sensing of drug-induced effects on the contractile machinery of human cardiomyocyte clusters. PLoS One. 2015;10:e0125540.
Krishnan R, Park JA, Seow CY, Lee PV, Stewart AG. Cellular biomechanics in drug screening and evaluation: mechanopharmacology. Trends Pharmacol Sci. 2016;37:87–100.
Zhou T, Marx KA, Dewilde AH, McIntosh D, Braunhut SJ. Dynamic cell adhesion and viscoelastic signatures distinguish normal from malignant human mammary cells using quartz crystal microbalance. Anal Biochem. 2012;421:164–71.
Steinem C, Janshoff A. Piezoelectric sensors. Berlin: Springer; 2007.
Muramatsu H, Tamiya E, Karube I. Computation of equivalent circuit parameters of quartz crystals in contact with liquids and study of liquid properties. Anal Chem. 1988;60:2142–6.
Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Method Enzymol. 2000;325:273–84.
Fischer-Friedrich E, Toyoda Y, Cattin CJ, Müller DJ, Hyman AA, Jülicher F. Rheology of the active cell cortex in mitosis. Biophys J. 2016;111(3):589–600.
Staunton JR, Doss BL, Lindsay S, Ros R. Correlating confocal microscopy and atomic force indentation reveals metastatic cancer cells stiffen during invasion into collagen I matrices. Sci Rep-UK. 2016;6:19686.
Silberberg YR, Mieda S, Amemiya Y, Sato T, Kihara T, Nakamura N, et al. Evaluation of the actin cytoskeleton state using an antibody-functionalized nanoneedle and an AFM. Biosens Bioelectron. 2013;40:3–9.
Hardin C, Rajendran K, Manomohan G, Tambe DT, Butler JP, Fredberg JJ, et al. Glassy dynamics, cell mechanics, and endothelial permeability. J Phys Chem B. 2013;117:12850–6.
Ren J, Huang H, Liu Y, Zheng X, Zou Q. An atomic force microscope study revealed two mechanisms in the effect of anticancer drugs on rate-dependent Young’s modulus of human prostate cancer cells. PLoS One. 2015;10:e0126107.
Wu HW, Kuhn T, Moy VT. Mechanical properties of L929 cells measured by atomic force microscopy: effects of anticytoskeletal drugs and membrane crosslinking. Scanning. 1998;20:389–97.
Al-Rekabi Z, Haase K, Pelling AE. Microtubules mediate changes in membrane cortical elasticity during contractile activation. Exp Cell Res. 2014;322:21–9.
Marx KA, Zhou T, Montrone A, McIntosh D, Braunhut SJ. A comparative study of the cytoskeleton binding drugs nocodazole and taxol with a mammalian cell quartz crystal microbalance biosensor: different dynamic responses and energy dissipation effects. Anal Biochem. 2007;361:77–92.
Acknowledgements
We thank Beijing Chenjing Electronic Co., Ltd. who provided the quartz crystal resonators and fabricated the eight HQCM chips used for this study; particularly, Mr. Lijun Zhao’s technical help is greatly appreciated. We also thank Prof. Dazhong Shen, College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, who lent us the Agilent 4395A impedance analyzer. Finally, we would like to thank Mr. Shuyue Zhou, Houston Methodist Hospital, USA, for proofreading the manuscript.
Funding
This work was supported in part by grants from the Key Project of Hunan Provincial Science & Technology Department (2013TT1009) and the National Natural Science Foundation of China (21275048).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shen, H., Zhou, T. & Hu, J. A high-throughput QCM chip configuration for the study of living cells and cell-drug interactions. Anal Bioanal Chem 409, 6463–6473 (2017). https://doi.org/10.1007/s00216-017-0591-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0591-4