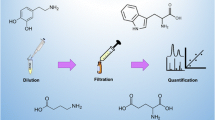
Abstract
The major monoamine neurotransmitters, serotonin (5-HT) and catecholamines (i.e., norepinephrine (NE), epinephrine (E), and dopamine (DA)), are critical to the nervous system function, and imbalances of the neurotransmitters have been connected to a variety of diseases, making their measurement useful in a clinical setting. A simple, rapid, robust, sensitive, and specific LC-MS/MS method has been developed and validated for the simultaneous quantitation of urinary serotonin and catecholamines with low cost, which is ideal for routine clinical applications. A simple extraction from complex urine was accomplished using tailored solid phase extraction incorporating phenylboronic acid complexation on a 96-well HLB microplate for the sample extraction and resulted in significantly improved throughput, selectivity, and extraction recovery. Compared to 1–10 mL of urine typically used, this method required only 10 μL. A rapid chromatographic elution with a total cycle time of 6 min per sample compared to reported run times of 19–75 min was achieved on a PFP column. The sensitivity of l and 2 ng mL−1 for the detection of low abundant E and NE combined with the high coverage of 1024 ng mL−1 for DA enabled the multi-analyte detection of these biogenic amines in a single run. Good linearity (2.0–512, 1.0–512, 4.0–1024, and 4.0–1024 ng mL−1 for NE, E, DA, and 5-HT, respectively), accuracy (87.6–104.0%), precision (≤8.0%), extraction recovery (69.6–103.7%), and matrix effect (87.1–113.1% for catecholamines and 63.6–71.4% for 5-HT) were obtained. No autosampler carryover was observed. The analytes were stable for 5 days at 20 °C, 14 days at 4 °C, and 30 days at −20 °C and five freeze–thaw cycles. The easy sample preparation, rapid LC, and multi-analyte MS detection allow two 96-well plates of samples to be extracted within 2 h and analyzed on an LC-MS/MS system within 24 h. The applicability and reliability of the assay were demonstrated by assessment of the reference interval for authentic urine specimens from 90 healthy individuals.

A simple, rapid, robust, sensitive and specific LC-MS/MS method combined with a dual functional solid phase extraction has been developed and validated for the simultaneous extraction and quantitation of monoamine neurotransmitters in human urine with low cost




Similar content being viewed by others
References
Ng J, Papandreou A, Heales SJ, Kurian MA. Monoamine neurotransmitter disorders—clinical advances and future perspectives. Nat Rev Neurol. 2015;11:567–84.
Marc DT, Ailts JW, Campeau DCA, Bull MJ, Olson KL. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: validity and clinical applicability. Neurosci Biobehav Rev. 2011;35:635–44.
Kema IP, de Vries EGE, Muskiet FAJ. Clinical chemistry of serotonin and metabolites. J Chromatogr B. 2000;747:33–48.
Goldstein DS. Catecholamines 101. Clin Auton Res. 2010;20:331–52.
Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem. 2014;60:1486–99.
Strac DS, Muck-Seler D, Pivac N. Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer’s disease: a review. Psychiatr Danub. 2015;27:14–24.
Dvorakova M, Jezova D, Blazicek P, Trebaticka J, Skodacek I, Suba J, et al. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): modulation by a polyphenolic extract from pine bark (pycnogenol). Nutr Neurosci. 2007;10:151–7.
Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57:353–8.
de Jong WHA, Wilkens MHLI, de Vries EGE, Kema IP. Automated mass spectrometric analysis of urinary and plasma serotonin. Anal Bioanal Chem. 2010;396:2609–16.
Nichkova MI, Huisman H, Wynveen PM, Marc DT, Olson KL, Kellermann GH. Evaluation of a novel ELISA for serotonin: urinary serotonin as a potential biomarker for depression. Anal Bioanal Chem. 2012;402:1593–600.
Masek K, Slánský J, Petrovický P, Hadden JW. Neuroendocrine immune interactions in health and disease. Int Immunopharmacol. 2003;3:1235–46.
Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51.
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol. 2016. doi:10.1002/jcp.25518.
Talwar D, Williamson C, Mclaughlin A, Gill A, O’Reilly DS. Extraction and separation of urinary catecholamines as their diphenyl boronate complexes using C18 solid-phase extraction sorbent and high-performance liquid chromatography. J Chromatogr B. 2002;769:341–9.
de Jong WHA, de Vries EGE, Wolffenbuttel BHR, Kema IP. Automated mass spectrometric analysis of urinary free catecholamines using on-line solid phase extraction. J Chromatogr B. 2010;878:1506–12.
Ji C, Walton J, Su Y, Tella M. Simultaneous determination of plasma epinephrine and norepinephrine using an integrated strategy of a fully automated protein precipitation technique, reductive ethylation labeling and UPLC-MS/MS. Anal Chim Acta. 2010;670:84–91.
Kumar A, Hart JP, McCalley DV. Determination of catecholamines in urine using hydrophilic interaction chromatography with electrochemical detection. J Chromatogr A. 2011;1218:3854–61.
Li XG, Li S, Kellermann G. An integrated liquid chromatography–tandem mass spectrometry approach for the ultra-sensitive determination of catecholamines in human peripheral blood mononuclear cells to assess neural-immune communication. J Chromatogr A. 2016;1449:54–61.
Ghasemi F, Hormozi-Nezhad MR, Mahmoudi M. Identification of catecholamine neurotransmitters using fluorescence sensor array. Anal Chim Acta. 2016;917:85–92.
Bicker J, Fortuna A, Alves G, Falcão A. Liquid chromatographic methods for the quantification of catecholamines and their metabolites in several biological samples—a review. Anal Chim Acta. 2013;768:12–34.
Saracino MA, Gerra G, Somaini L, Colombati M, Raggi MA. Chromatographic analysis of serotonin, 5-hydroxyindolacetic acid and homovanillic acid in dried blood spots and platelet poor and rich plasma samples. J Chromatogr A. 2010;1217:4808–14.
Perry M, Li Q, Kennedy RT. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal Chim Acta. 2009;653:1–22.
Greco S, Danysz W, Zivkovic A, Gross R, Stark H. Microdialysate analysis of monoamine neurotransmitters—a versatile and sensitive LC-MS/MS method. Anal Chim Acta. 2013;771:65–72.
Tang YB, Sun F, Teng L, Li WB, An SM, Zhang C, et al. Simultaneous determination of the repertoire of classical neurotransmitters released from embryonal carcinoma stem cells using online microdialysis coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal Chim Acta. 2014;849:70–9.
de Jong WHA, de Vries EGE, Kema IP. Current status and future developments of LC-MS/MS in clinical chemistry for quantification of biogenic amines. Clin Biochem. 2011;44:95–103.
Miekus N, Baczek T. Non-invasive screening for neuroendocrine tumors—biogenic amines as neoplasm biomarkers and the potential improvement of “gold standards”. J Pharm Biomed Anal. 2016;130:194–201.
Gosetti F, Mazzucco E, Gennaro MC, Marengo E. Simultaneous determination of sixteen underivatized biogenic amines in human urine by HPLC-MS/MS. Anal Bioanal Chem. 2013;405:907–16.
Li XG, Li S, Wynveen P, Mork K, Kellermann G. Development and validation of a specific and sensitive LC-MS/MS method for quantification of urinary catecholamines and application in biological variation studies. Anal Bioanal Chem. 2014;406:7287–97.
Park NH, Hong JY, Shin HJ, Hong J. Comprehensive profiling analysis of bioamines and their acidic metabolites in human urine by gas chromatography/mass spectrometry combined with selective derivatization. J Chromatogr A. 2013;1305:234–43.
Naccarato A, Gionfriddo E, Sindona G, Tagarelli A. Development of a simple and rapid solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry method for the analysis of dopamine, serotonin and norepinephrine in human urine. Anal Chim Acta. 2014;810:17–24.
Jiang L, Chen Y, Chen Y, Ma M, Tan Y, Tang H, et al. Determination of monoamine neurotransmitters in human urine by carrier-mediated liquid-phase microextraction based on solidification of stripping phase. Talanta. 2015;144:356–62.
Yang X, Hu Y, Li G. Online micro-solid-phase extraction based on boronate affinity monolithic column coupled with high-performance liquid chromatography for the determination of monoamine neurotransmitters in human urine. J Chromatogr A. 2014;1342:37–43.
Konieczna L, Roszkowska A, Niedzwiecki M, Baczek T. Hydrophilic interaction chromatography combined with dispersive liquid-liquid microextraction as a preconcentration tool for the simultaneous determination of the panel of underivatized neurotransmitters in human urine samples. J Chromatogr A. 2016;1431:111–21.
Bouri M, Lerma-García MJ, Salghi R, Zougagh M, Ríos A. Selective extraction and determination of catecholamines in urine samples by using a dopamine magnetic molecularly imprinted polymer and capillary electrophoresis. Talanta. 2012;99:897–903.
Tsunoda M. Recent advances in methods for the analysis of catecholamines and their metabolites. Anal Bioanal Chem. 2006;386:506–14.
Putman DF. Composition and concentrative properties of human urine; 1971. NASA Contract Rep 109.
Li XG, Li S, Kellermann G. Pre-analytical and analytical validations and clinical applications of a miniaturized, simple and cost-effective solid phase extraction combined with LC-MS/MS for the simultaneous determination of catecholamines and metanephrines in spot urine samples. Talanta. 2016;159:238–47.
Roberts NB, Higgins G, Sargazi M. A study on the stability of urinary free catecholamines and free methyl-derivatives at different pH, temperature and time of storage. Clin Chem Lab Med. 2010;48:81–7.
Clinical and Laboratory Standards Institute. Liquid chromatography-mass spectrometry methods; approved guidance, CLSI document C62-A; 2014.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30.
Acknowledgements
The authors acknowledge Pheng Yang for providing technical assistance and Melissa Ahrens for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All clinical studies involving human urine samples were conducted in adherence to the procedure approved by Pharmasan/Neuroscience Institutional Review Board (IRB).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 217 kb)
Rights and permissions
About this article
Cite this article
Li, X.(., Li, S. & Kellermann, G. Simultaneous extraction and determination of monoamine neurotransmitters in human urine for clinical routine testing based on a dual functional solid phase extraction assisted by phenylboronic acid coupled with liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 409, 2859–2871 (2017). https://doi.org/10.1007/s00216-017-0231-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0231-z