Abstract
Metabolomics protocols are used to comprehensively characterize the metabolite content of biological samples by exploiting cutting-edge analytical platforms, such as gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry (MS) assays, as well as nuclear magnetic resonance (NMR) assays. We have developed novel sample preparation procedures combined with GC-MS, LC-MS, and NMR metabolomics profiling for analyzing bronchial wash (BW) and bronchoalveolar lavage (BAL) fluid from 15 healthy volunteers following exposure to biodiesel exhaust and filtered air. Our aim was to investigate the responsiveness of metabolite profiles in the human lung to air pollution exposure derived from combustion of biofuels, such as rapeseed methyl ester biodiesel, which are increasingly being promoted as alternatives to conventional fossil fuels. Our multi-platform approach enabled us to detect the greatest number of unique metabolites yet reported in BW and BAL fluid (82 in total). All of the metabolomics assays indicated that the metabolite profiles of the BW and BAL fluids differed appreciably, with 46 metabolites showing significantly different levels in the corresponding lung compartments. Furthermore, the GC-MS assay revealed an effect of biodiesel exhaust exposure on the levels of 1-monostearylglycerol, sucrose, inosine, nonanoic acid, and ethanolamine (in BAL) and pentadecanoic acid (in BW), whereas the LC-MS assay indicated a shift in the levels of niacinamide (in BAL). The NMR assay only identified lactic acid (in BW) as being responsive to biodiesel exhaust exposure. Our findings demonstrate that the proposed multi-platform approach is useful for wide metabolomics screening of BW and BAL fluids and can facilitate elucidation of metabolites responsive to biodiesel exhaust exposure.

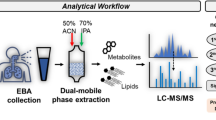
Graphical abstract illustrating the study workflow. NMR Nuclear Magnetic Resonance, LC-TOFMS Liquid chromatography-Time Of Flight Mass Spectrometry, GC Gas Chromatography-Mass spectrometry




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CV-ANOVA:
-
Cross-validation-analysis of variance
- GC-MS:
-
Gas chromatography-mass spectrometry
- LC-MS:
-
Liquid chromatography-mass spectrometry
- MVA:
-
Multivariate analysis
- NMR:
-
Nucleic magnetic resonance
- OPLS:
-
Orthogonal projections to latent structures
- OPLS-DA:
-
Orthogonal projections to latent structures-discriminant analysis
- PCA:
-
Principal component analysis
- RME:
-
Rapeseed methyl ester
- ROC:
-
Receiver operating characteristic
- RSD:
-
Relative standard deviations
- SMC:
-
Swedish Metabolomics Centre
- UPSC:
-
Umeå Plant Science Centre
References
Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26(1):51–78.
Ellis DI et al. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics. 2007;8(9):1243–66.
Evans CR et al. Untargeted LC–MS Metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J Proteome Res. 2014;13(2):640–9.
Ho WE et al. Metabolomics reveals altered metabolic pathways in experimental asthma. Am J Respir Cell Mol Biol. 2013;48(2):204–11.
Xu Y-J et al. Recent developments and applications of metabolomics in microbiological investigations. TrAC Trends Anal Chem. 2014;56:37–48.
Larive CK, Barding GA, Dinges MM. NMR Spectroscopy for metabolomics and metabolic profiling. Anal Chem. 2015;87(1):133–46.
Karimpour M et al. Postprandial metabolomics: a pilot mass spectrometry and NMR study of the human plasma metabolome in response to a challenge meal. Anal Chim Acta. 2016;908:121–31.
Walters EH, Ward C, Li X. Bronchoalveolar lavage in asthma research. Respirology. 1996;1(4):233–45.
Peng J et al. Metabolomic profiling of bronchoalveolar lavage fluids by isotope labeling liquid chromatography mass spectrometry: a promising approach to studying experimental asthma. Metabolomics. 2014;10(6):1305–17.
Singh C et al. Mini-bronchoalveolar lavage fluid can be used for biomarker identification in patients with lung injury by employing (1)H NMR spectroscopy. Crit Care. 2013;17(2):430.
Connett GJ. Bronchoalveolar lavage. Paediatr Respir Rev. 2000;1(1):52–6.
Hu JZ et al. Metabolomics in lung inflammation: a high resolution (1)h NMR study of mice exposed to silica dust. Toxicol Mech Methods. 2008;18(5):385–98.
Ho WE et al. Anti-malarial drug artesunate restores metabolic changes in experimental allergic asthma. Metabolomics. 2014;11(2):380–90.
Wolak JE, Esther CR, O’Connell TM. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers. 2009;14(1):55–60.
Fabiano A et al. Metabolomic analysis of bronchoalveolar lavage fluid in preterm infants complicated by respiratory distress syndrome: preliminary results. J Matern Fetal Neonatal Med. 2011;24(S2):55–8.
Julák J et al. Bronchoalveolar lavage examined by solid phase microextraction, gas chromatography–mass spectrometry and selected ion flow tube mass spectrometry. J Microbiol Methods. 2006;65(1):76–86.
Rai R et al. Metabolic profiling in human lung injuries by high-resolution nuclear magnetic resonance spectroscopy of bronchoalveolar lavage fluid (BALF). Metabolomics. 2013;9(3):667–76.
Ho WE et al. Metabolomics reveals inflammatory-linked pulmonary metabolic alterations in a murine model of house dust mite-induced allergic asthma. J Proteome Res. 2014;13(8):3771–82.
Larsson N et al. Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans. Eur Respir J. 2014;43(2):453–63.
Brook RD et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–67.
Lucking AJ et al. Diesel exhaust inhalation increases thrombus formation in man†. Eur Heart J. 2008;29(24):3043–51.
Törnqvist H et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176(4):395–400.
Löndahl J et al. Measurement techniques for respiratory tract deposition of airborne nanoparticles: a critical review. J Aerosol Med Pulm Drug Deliv. 2013;27(4):229–54.
Sivagangabalan G et al. The effect of air pollution on spatial dispersion of myocardial repolarization in healthy human volunteers. J Am Coll Cardiol. 2011;57(2):198–206.
Mills NL et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–6.
Mills NL et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–82.
Lundbäck M et al. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol. 2009;6:7–7.
Barath S et al. Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol. 2010;7(1):1–11.
Lucking AJ et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123(16):1721–8.
Mills NL et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32(21):2660–71.
Hoek G et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;12(1):43.
Pope CA, Ezzati M, Dockery DW. Fine particulate air pollution and US county life expectancies. N Engl J Med. 2009;360(4):376–86.
Brook RD et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78.
Thibodeau LA et al. Air pollution and human health: a review and reanalysis. Environ Health Perspect. 1980;34:165–83.
Langrish JP et al. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J Intern Med. 2012;272(3):224–39.
Robbins M. Policy: fuelling politics. Nature. 2011;474(7352):S22–4.
Bünger J et al. Potential hazards associated with combustion of bio-derived versus petroleum-derived diesel fuel. Crit Rev Toxicol. 2012;42(9):732–50.
Salvi S et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159(3):702–9.
A J et al. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal Chem. 2005;77(24):8086–94.
Hong J-H et al. Characterization of the biochemical effects of naphthalene on the mouse respiratory system using NMR-based metabolomics. J Appl Toxicol. 2014;34(12):1379–88.
Beckonert O et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2(11):2692–703.
Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemom. 2002;16(3):119–28.
Eriksson, L., Multi- and Megavariate Data Analysis. 2006: Umetrics AB.
Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37(1):36–48.
Egan JP. Signal detection theory and ROC analysis. Series in Cognition and Perception; New York: Academic; 1975.
Cribbs SK et al. Metabolomics of bronchoalveolar lavage differentiate healthy HIV-1-infected subjects from controls. AIDS Res Hum Retrovir. 2014;30(6):579–85.
Xia J et al. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40(Web Server issue):W127–33.
Want EJ et al. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal Chem. 2006;78(3):743–52.
Crews B et al. Variability analysis of human plasma and cerebral spinal fluid reveals statistical significance of changes in mass spectrometry-based metabolomics data. Anal Chem. 2009;81(20):8538–44.
Masson P et al. Optimization and evaluation of metabolite extraction protocols for untargeted metabolic profiling of liver samples by UPLC-MS. Anal Chem. 2010;82(18):7779–86.
Egan JP. Signal detection theory and roc-analysis. New York: Academic press; 1975.
Acknowledgments
Financial support from The Swedish Research Council Formas, the Swedish Heart Lung Foundation, Västerbotten County Council (Spjutspetsmedel), AFA-Insurance, and the Kempe Foundation is gratefully acknowledged. The Swedish Metabolomics Centre (www.swedishmetabolomicscentre.se) is acknowledged for outstanding assistance with the analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Izabella Surowiec and Masoumeh Karimpour contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2377 kb)
Rights and permissions
About this article
Cite this article
Surowiec, I., Karimpour, M., Gouveia-Figueira, S. et al. Multi-platform metabolomics assays for human lung lavage fluids in an air pollution exposure study. Anal Bioanal Chem 408, 4751–4764 (2016). https://doi.org/10.1007/s00216-016-9566-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9566-0