Abstract
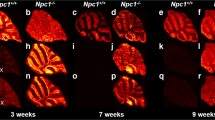
Mucopolysaccharidosis type II (Hunter’s disease) mouse model (IdS-KO) was investigated by both imaging mass spectrometry (IMS) and immunohistochemistry (IHC) performed on the same tissue sections. For this purpose, IdS-KO mice brain sections were coated with sublimated 1,5-diaminonaphtalene and analyzed by high spatial resolution IMS (5 μm) and anti-GM3 IHC on the same tissue sections to characterize the ganglioside monosialated ganglioside (GM) deposits found in Hunter’s disease. IMS analysis have found that two species of GM3 and GM2 that are only different due to the length of their fatty acid residue (stearic or arachidic residue) were overexpressed in the IdS-KO mice compared to a control mouse. GM3 and GM2 were characterized by on-tissue exact mass and MS/MS compared to a GM3 standard. Realignment of both IMS and IHC data sets further confirmed the observed regioselective signal previously detected by providing direct correlation of the IMS image for the two GM3 overly expressed MS signals with the anti-GM3 IHC image. Furthermore, these regioselective GM MS signals were also found to have highly heterogeneous distributions within the GM3-IHC staining. Some deposits showed high content in GM3 and GM2 stearic species (r = 0.74) and others had more abundant GM3 and GM2 arachidic species (r = 0.76). Same-section analysis of Hunter’s disease mouse model by both high spatial resolution IMS and IHC provides a more in-depth analysis of the composition of the GM aggregates while providing spatial distribution of the observed molecular species.

Ganglioside imaging mass spectrometry followed by immunohistochemistry performed on the same tissue section





Similar content being viewed by others
REFERENCES
Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, editor. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. p. 3421–52.
Fratanto JC, Hall CW, Neufeld EF. Defect in Hurlers and Hunters syndromes—faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968;60(2):699. doi:10.1073/pnas.60.2.699.
Walkley SU. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin Cell Dev Biol. 2004;15(4):433–44. doi:10.1016/j.semcdb.2004.03.002.
Walkley SU, Vanier MT. Secondary lipid accumulation in lysosomal disease. Biochim Biophys Acta (BBA) Mol Cell Res. 2009;1793(4):726–36. doi:10.1016/j.bbamcr.2008.11.014.
Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med. 2006;8(8):465–73. doi:10.1097/01.gim.0000232477.37660.fb.
Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, et al. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167(3):267–77. doi:10.1007/s00431-007-0635-4.
Muenzer J, Fu H. Targeted disruption of the mouse iduronate sulfatase gene. Am J Hum Genet. 1999;65(4):A427–A.
Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr. 2002;91:98–9. doi:10.1080/080352502762458012.
Jung S-C, Park E-S, Choi EN, Kim CH, Kim SJ, Jin D-K. Characterization of a novel mucopolysaccharidosis type II mouse model and recombinant AAV2/8 vector-mediated gene therapy. Mol Cells. 2010;30(1):13–8. doi:10.1007/s10059-010-0083-2.
Friso A, Tomanin R, Alba S, Gasparotto N, Puicher EP, Fusco M, et al. Reduction of GAG storage in MPS II mouse model following implantation of encapsulated recombinant myoblasts. J Gene Med. 2005;7(11):1482–91. doi:10.1002/jgm.790.
McGlynn R, Dobrenis K, Walkley SU. Differential subcellular localization of cholesterol, gangliosides, and glycosaminoglycans in murine models of mucopolysaccharide storage disorders. J Comp Neurol. 2004;480(4):415–26. doi:10.1002/cne.20355.
Kreutz F, Petry FS, Camassola M, Schein V, Guma FCR, Nardi NB. Alterations of membrane lipids and in gene expression of ganglioside metabolism in different brain structures in a mouse model of mucopolysaccharidosis type I (MPS I). Gene. 2013;527(1):109–14. doi:10.1016/j.gene.2013.06.002.
Walkley SU. Pathogenic mechanisms in lysosomal disease: a reappraisal of the role of the lysosome. Acta Paediatr. 2007;96:26–32. doi:10.1111/j.1651-2227.2007.00202.x.
Han XL, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24(3):367–412. doi:10.1002/mas.20023.
Borrull A, López-Martínez G, Poblet M, Cordero-Otero R, Rozès N. A simple method for the separation and quantification of neutral lipid species using GC-MS. Eur J Lipid Sci Technol. 2015;117(3):274–80. doi:10.1002/ejlt.201400064.
Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CHL. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010;121(3-5):496–504. doi:10.1016/j.jsbmb.2010.04.010.
Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. 2001;7(4):493–6. doi:10.1038/86573.
Patterson NH, Doonan RJ, Daskalopoulou SS, Dufresne M, Lenglet S, Montecucco F, et al. Three-dimensional imaging MS of lipids in atherosclerotic plaques: open-source methods for reconstruction and analysis. Proteomics 2016:1642–51. doi:10.1002/pmic.201500490.
Chaurand P, Schwartz SA, Billheimer D, Xu BGJ, Crecelius A, Caprioli RM. Integrating histology and imaging mass spectrometry. Anal Chem. 2004;76(4):1145–55. doi:10.1021/ac0351264.
Yu RK, Tsai YT, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides—an overview. J Oleo Sci. 2011;60(10):537–44. doi:10.5650/jos.60.537.
Chen YF, Allegood J, Liu Y, Wang E, Cachon-Gonzalez B, Cox TM, et al. Imaging MALDI mass spectrometry using an oscillating capillary nebulizer matrix coating system and its application to analysis of lipids in brain from a mouse model of Tay-Sachs/Sandhoff disease. Anal Chem. 2008;80(8):2780–8. doi:10.1021/ac702350g.
Chan K, Lanthier P, Liu X, Sandhu JK, Stanimirovic D, Li JJ. MALDI mass spectrometry imaging of gangliosides in mouse brain using ionic liquid matrix. Anal Chim Acta. 2009;639(1-2):57–61. doi:10.1016/j.aca.2009.02.051.
Colsch B, Woods AS. Localization and imaging of sialylated glycosphingolipids in brain tissue sections by MALDI mass spectrometry. Glycobiology. 2010;20(6):661–7. doi:10.1093/glycob/cwq031.
Weishaupt N, Caughlin S, Yeung KKC, Whitehead SN. Differential anatomical expression of ganglioside GM1 species containing d18:1 or d20:1 sphingosine detected by MALDI imaging mass spectrometry in mature rat brain. Front Neuroanat. 2015;9. doi:10.3389/fnana.2015.00155.
Zavalin A, Todd EM, Rawhouser PD, Yang JH, Norris JL, Caprioli RM. Direct imaging of single cells and tissue at sub-cellular spatial resolution using transmission geometry MALDI MS. J Mass Spectrom. 2012;47(11):1473–81. doi:10.1002/jms.3108.
Zavalin A, Yang J, Hayden K, Vestal M, Caprioli RM. Tissue protein imaging at 1 μm laser spot diameter for high spatial resolution and high imaging speed using transmission geometry MALDI TOF MS. Anal Bioanal Chem. 2015;407(8):2337–42. doi:10.1007/s00216-015-8532-6.
McDonnell LA, Heeren RMA. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26(4):606–43. doi:10.1002/mas.20124.
Thomas A, Charbonneau JL, Fournaise E, Chaurand P. Sublimation of new matrix candidates for high spatial resolution imaging mass spectrometry of lipids: enhanced information in both positive and negative polarities after 1,5-diaminonapthalene deposition. Anal Chem. 2012;84(4):2048–54. doi:10.1021/ac2033547.
Vanhoof F, Hers HG. Abnormalities of lysosomal enzymes in mucopolysaccharidoses. Eur J Biochem. 1968;7(1):34.
Abdelmoula WM, Carreira RJ, Shyti R, Balluff B, van Zeijl RJM, Tolner EA, et al. Automatic registration of mass spectrometry imaging data sets to the Allen Brain Atlas. Anal Chem. 2014;86(8):3947–54. doi:10.1021/ac500148a.
Heijs B, Abdelmoula WM, Lou S, Briaire-de Bruijn IH, Dijkstra J, Bovée JVMG, et al. Histology-guided high-resolution matrix-assisted laser desorption ionization mass spectrometry imaging. Anal Chem. 2015;87(24):11978–83. doi:10.1021/acs.analchem.5b03610.
Acknowledgements
The authors acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Foundation for Innovation (CFI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1499 kb)
Rights and permissions
About this article
Cite this article
Dufresne, M., Guneysu, D., Patterson, N.H. et al. Multimodal detection of GM2 and GM3 lipid species in the brain of mucopolysaccharidosis type II mouse by serial imaging mass spectrometry and immunohistochemistry. Anal Bioanal Chem 409, 1425–1433 (2017). https://doi.org/10.1007/s00216-016-0076-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0076-x