Abstract
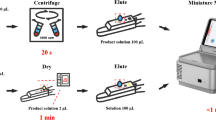
Glycosylated hemoglobin A1c (HbA1c) is a useful marker for the diagnosis of diabetes mellitus. Commercial column separation methods for HbA1c measurement were lacking throughput and sometimes interfered with hemoglobin variants. In this work, we developed a high-throughput and specific method for HbA1c by quantitative measurement of N-terminal peptides (NT method). Two thousand specimens could be measured in 8 h. The high-throughput was achieved by using a fast analysis of matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) and an efficient proteolysis accelerated by laser irradiation. An intensity ratio of glycosylated to non-glycosylated hemoglobin N-terminal peptides was used to calculate the HbA1c level in blood. Interference from Hb variants of N-terminal peptides could be excluded by a highly accurate mass selection. The coefficient of variation (CV) of intra-assay precision was 9.8 and 9.9 %, respectively. The CVs of inter-assay precision over 20 days were 9.1 and 8.4 %, respectively. Measurement results were well correlated with the commercially available column method (r = 0.995). The NT method is promising for large-scale screening for diabetes mellitus among people.




Similar content being viewed by others
References
Bunn HF, Haney DN, Kamin S, Gabbay KH, Gallop PM. Biosynthesis of human hemoglobin A1C-slow glycosylation of hemoglobin invivo. J Clin Invest. 1976;57(6):1652–9.
Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–72.
John WG. Haemoglobin A(1c): analysis and standardisation. Clin Chem Lab Med. 2003;41(9):1199–212.
Hartog JWL, Voors AA, Bakker SJL, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGES) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9(12):1146–55.
Perneger TV, Brancati FL, Whelton PK, Klag MJ. End-stage renal-disease attributable to diabetes-mellitus. Ann Inter Med. 1994;121(12):912–8.
Turk Z, Misur I, Turk N. Temporal association between lens protein glycation and cataract development in diabetic rats. Acta Diabetol. 1997;34(1):49–54.
Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes-epdemiology and prevention. Diabetes Care. 1989;12(1):24–31.
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21.
Garlick RL, Mazer JS, Higgins PJ, Bunn HF. Characterization of glycosylated hemoglobins-relelevance to monitoring of diabetic control and analysis of other proteins. J Clin Invest. 1983;71(5):1062–71.
Peterson KP, Pavlovich JG, Goldstein D, Little R, England J, Peterson CM. What is hemoglobin Alc? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem. 1998;44(9):1951–8.
Turpeinen U, Karjalainen U, Stenman UH. 3 assays-for glycohemoglobin compared. Clin Chem. 1995;41(2):191–5.
Mallia AK, Hermanson GT, Krohn RI, Fujimoto EK, Smith PK. Preparation and use of a boronic acid affinity support for separation and quantitation of glycosylated hemoglobins. Anal Lett. 1981;14(8):649–61.
Klenk DC, Hermanson GT, Krohn RI, Fujimoto EK, Mallia AK, Smith PK, et al. Determination of glycosylated hemoglobin by affinity-chromatography-comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin Chem. 1982;28(10):2088–94.
Mack S, Cruzado-Park I, Chapman J, Ratnayake C, Vigh G. A systematic study in CIEF: defining and optimizing experimental parameters critical to method reproducibility and robustness. Electrophoresis. 2009;30(23):4049–58.
Hageman JH, Kuehn GD. Assay of adenylate-cyclase by use of polyacrylamide-boronate gel columns. Anal Biochem. 1977;80(2):547–54.
Turner APF, Chen BN, Piletsky SA. In vitro diagnostics in diabetes: meeting the challenge. Clin Chem. 1999;45(9):1596–601.
Gallop PM, Fluckiger R, Hanniken A, Mininsohn MM, Gabbay KH. Chemical quantitation of hemoglobin glycosylation-fluoromeetric detection of formaldehyde released upon periodate-oxidation of glycoglobin. Anal Biochem. 1981;117(2):427–32.
Fluckiger R, Winterhalter KH. Invitro synthesis of hemoglobin a1c. FEBS Lett. 1976;71(2):356–60.
Pundir CS, Chawla S. Determination of glycated hemoglobin with special emphasis on biosensing methods. Anal Biochem. 2014;444:47–56.
Torke NS, Boral L, Nguyen T, Chakrin A, Kimball D. Comparison of four methods for glycohemoglobin (HbA1c) determination. Clin Chem. 2005;51(6):A242–3.
Roberts WL, Safar-Pour S, De BK, Rohlfing CL, Weykamp CW, Little RR. Effects of hemoglobin C and S traits on glycohemoglobin measurements by eleven methods. Clin Chem. 2005;51(4):776–8.
Roberts WL, De BK, Hanbury C, Hoyer JD, Rohlfing C, Little RR. Effects of hemoglobin C and S traits on eight glycohemoglobin methods. Clin Chem. 2002;48(2):383–5.
Weykamp CW, Martina WV, VanderDijs FPL, Penders TJ, Vanderslik W, Muskiet FAJ. Hemoglobin-s and hemoglobin-c—reference values for glycohemoglobin in heterozygous, double-heterozygous and homozygous subjects, as established by 13 methods. Clin Chim Acta. 1994;231(2):161–71.
Lorenzo-Medina M, De-La-Iglesia S, Ropero P, Martin-Alfaro R, Quintana-Hidalgo L. Interference of hemoglobin D on measurements of hemoglobin A1c by the high-performance liquid chromatography HA-8160 in 27 patients. J Diabetes Sci Technol. 2012;6(5):1235–7.
Fluckiger R, Woodtli T, Berger W. Quantitation of glycosylated hemoglobin by boronate affinity-chromatography. Diabetes. 1984;33(1):73–6.
Johnson RN, Baker JR. Inaccuracy in measuring glycated albumin comcentration by thiobarbituric acid colorimetry and by boronate chromatography. Clin Chem. 1988;34(7):1456–9.
Lin CN, Emery TJ, Little RR, Hanson SE, Rohlfing CL, Jaissonc S. Effects of hemoglobin C, D, E, and S traits on measurements of HbA(1c) by six methods. Clin Chim Acta. 2012;413(7-8):819–21.
Little RR, Rohlfing CL, Hanson S, Connolly S, Higgins T, Weykamp CW, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA(1c)) by 23 methods. Clin Chem. 2008;54(8):1277–82.
Jeppsson J, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40(1):78–89.
Szajli E, Feher T, Medzihradszky KF. Investigating the quantitative nature of MALDI-TOF MS. Mol Cell Proteomics. 2008;7(12):2410–8.
Liu SS, Chen HM, Lu XH, Deng CH, Zhang XM, Yang PY. Facile synthesis of copper(II) immobilized on magnetic mesoporous silica microspheres for selective enrichment of peptides for mass spectrometry analysis. Angew Chem Int Ed. 2010;49(41):7557–61.
Stolowitz ML. On-target and nanoparticle-facilitated selective enrichment of peptides and proteins for analysis by MALDI-MS. Proteomics. 2012;12(23-24):3438–50.
Zhang XY, Zhu SC, Xiong Y, Deng CH, Zhang XM. Development of a MALDI-TOF MS strategy for the high-throughput analysis of biomarkers: on-target aptamer immobilization and laser-accelerated proteolysis. Angew Chem Int Ed. 2013;52(23):6055–8.
Yao GP, Deng CH, Zhang XM, Yang PY. Efficient tryptic proteolysis accelerated by laser radiation for peptide mapping in proteome analysis. Angew Chem Int Ed. 2010;49(44):8185–9.
Lin CN, Emery TJ. Effects of hemoglobin C, D, E, and S traits on measurements of HbA(1c) by six methods. Clin Chim Acta. 2012;413(7-8):819–21.
Weykamp C, Visser-Dekkers W, Kemna E, Siebelder C. Effects of hemoglobin D and E on the measurement of HbA(1c) with the modified Menarini/ARKRAY ADAMS A1c HA-8180V analyser. Clin Chim Acta. 2012;414:44–5.
Kleifeld O, Doucet A, Prudova A, Keller UAD, Gioia M, Kizhakkedathu JN, et al. Identifying and quantifying proteolytic events and the natural N terminome by terminal amine isotopic labeling of substrates. Nat Protoc. 2011;6(10):1578–611.
Kleifeld O, Doucet A, Keller UAD, Prudova A, Schilling O, Kainthan RK, et al. Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat Biotechnol. 2010;28(3):281–8.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (project 21175026) and the National Research Projects of China (2012AA020202, 2012CB910604, 2013CB911201, and 2012YQ12004409).
Author contributions
All authors have given approval to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
The research followed the tenets of the Declaration of Helsinki and the use of these blood samples for research was approved by the Ethics Committee of Zhongshan Hospital, Fudan University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 104 kb)
Rights and permissions
About this article
Cite this article
Li, L., Zang, W. & Zhang, X. A high-throughput method for measurement of glycohemoglobin in blood samples utilizing laser-accelerated proteolysis and MALDI-TOF MS. Anal Bioanal Chem 408, 1507–1513 (2016). https://doi.org/10.1007/s00216-015-9258-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9258-1