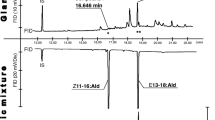
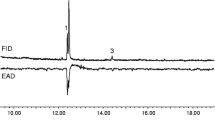
Abstract
Mating disruption is a sustainable method for the control of insect pests, involving the release of synthetic sex pheromones that disrupt the olfactory localization of females by males. However, the development and refinement of this strategy is hampered because current instruments lack the sensitivity to detect volatile organic chemicals in the field, and portable electroantennograms produce non-comparable relative units and distorted results in the presence of plant volatiles. To address the demand for more sensitive instruments that are suitable for the rapid in situ detection of airborne pheromones, we have developed a portable, automated needle trap device connected to a gas chromatograph, mass spectrometer, and electroantennographic detector (NTD-GC-MS/EAD) suitable for field applications. We tested the instrument by measuring the concentration of the sex pheromone (E,Z)-7,9-dodecadienyl acetate, which is used to disrupt the mating of the European grapevine moth Lobesia botrana (Lepidoptera: Tortricidae). Our data confirm that the instrument generates highly reproducible results and is highly sensitive, with a detection threshold of 3 ng/m3 (E,Z)-7,9-dodecadienyl acetate in outside air.








Similar content being viewed by others
References
Pimentel D (2009) Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. In: Peshin R, Dhawan A (eds) Integrated Pest Management: Innovation-Development Process. Springer Netherlands, Amsterdam, pp 89–111. doi:10.1007/978-1-4020-8992-3_4
McNeil JN, Cotnoir PA, Leroux T, Laprade R, Schwartz JL (2010) A Canadian national survey on the public perception of biological control. Biocontrol 55(4):445–454. doi:10.1007/s10526-010-9273-2
Gonzalez-Rodriguez RM, Rial-Otero R, Cancho-Grande B, Gonzalez-Barreiro C, Simal-Gandara J (2011) A Review on the Fate of Pesticides during the Processes within the Food-Production Chain. Crit Rev Food Sci 51(2):99–114. doi:10.1080/10408390903432625
Witzgall P, Kirsch P, Cork A (2010) Sex Pheromones and Their Impact on Pest Management. J Chem Ecol 36(1):80–100. doi:10.1007/s10886-009-9737-y
Tette JP (1974) Pheromones in insect population management. In: Birch MC (ed) Pheromones. North-Holland Publishing Company, Amsterdam, pp 399–410
Sauer AE, Karg G, Koch UT, Dekramer JJ, Milli R (1992) A Portable EAG System for the Measurement of Pheromone Concentrations in the Field. Chem Senses 17(5):543–553. doi:10.1093/chemse/17.5.543
Flint HM, Yamamoto AK, Parks NJ, Nyomura K (1993) Aerial Concentrations of Gossyplure, the Sex-Pheromone of the Pink-Bollworm (Lepidoptera, Gelechiidae), within and above Cotton Fields Treated with Long-Lasting Dispensers. Environ Entomol 22(1):43–48
Baker TC, Haynes KF (1989) Field and Laboratory Electroantennographic Measurements of Pheromone Plume Structure Correlated with Oriental Fruit Moth Behavior. Physiol Entomol 14(1):1–12. doi:10.1111/j.1365-3032.1989.tb00931.x
Milli R, De Kramer JJ (1989) An EAG detector for field pheromone measurements. In: Elmer R, Singer W (eds) Dynamics and Plasticity in Neuronal Systems: Proceedings of the 17th Göttingen Neurobiology Conference. Thieme, Stuttgart, p 77
Schütz S, Weissbecker B, Hummel HE (1996) Biosensor for volatiles released by damaged plants. Biosens Bioelectron 11(4):427–433. doi:10.1016/0956-5663(96)82738-2
Rumbo ER, Suckling DM, Karg G (1995) Measurement of Airborne Pheromone Concentrations Using Electroantennograms - Interactions between Environmental Volatiles and Pheromone. J Insect Physiol 41(6):465–471. doi:10.1016/0022-1910(94)00133-2
Koch UT, Carde AM, Carde RT (2002) Calibration of an EAG system to measure airborne concentration of pheromone formulated for mating disruption of the pink bollworm moth, Pectinophora gossypiella (Saunders) (Lep., Gelechiidae). J Appl Entomol 126(7–8):431–435. doi:10.1046/j.1439-0418.2002.00673.x
Karg G, Sauer AE (1995) Spatial Distribution of Pheromone in Vineyards Treated for Mating Disruption of the Grape Vine Moth Lobesia botrana Measured with Electroantennograms. J Chem Ecol 21(9):1299–1314. doi:10.1007/Bf02027563
Karg G, Suckling DM, Bradley SJ (1997) Defining interaction between electroantennogram responses of Epiphyas postvittana (Lepidoptera: Tortricidae) to pheromone and other volatiles. J Insect Physiol 43(2):179–187. doi:10.1016/S0022-1910(96)00083-2
Schöning MJ, Koch UT, Schütz S (2002) Analyseverfahren zur schnellen und hochempfindlichen Registrierung von Duftstoffgemischen in der Atmosphäre. PCT/DE97/01494, 21.08.2002
Rützler M, Zwiebel LJ (2005) Molecular biology of insect olfaction: recent progress and conceptual models. J Comp Physiol A 191(9):777–790. doi:10.1007/s00359-005-0044-y
Hansson BS, Stensmyr MC (2011) Evolution of Insect Olfaction. Neuron 72(5):698–711. doi:10.1016/j.neuron.2011.11.003
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol 58:373–391. doi:10.1146/annurev-ento-120811-153635
Leal WS (2005) Pheromone reception. Top Curr Chem 240:1–36. doi:10.1007/B98314
Arn H, Stadler E, Rauscher S (1975) The Electroantennagraphic Detector - A Selective and Sensitive Tool in Gas Chromatographic Analysis of Insect Pheromones. Z Naturforsch C 30(6):722–725
Struble DL, Arn H (1984) Combined Gas Chromatography and Electroantennogram Recording of Insect Olfactory Responses. In: Hummel H, Miller T (eds) Techniques in Pheromone Research. Springer Series in Experimental Entomology. Springer, New York, pp 161–178
Arn H, Rauscher S, Buser HR, Roelofs WL (1976) Sex-Pheromone of Eupoecilia ambiguella: cis-9-Dodecenyl Acetate as a Major Component. Z Naturforsch C 31(9–10):499–503
Witzgall P, Tasin M, Buser HR, Wegner-Kiss G, Mancebon VSM, Ioriatti C, Backman AC, Bengtsson M, Lehmann L, Francke W (2005) New pheromone components of the grapevine moth Lobesia botrana. J Chem Ecol 31(12):2923–2932. doi:10.1007/s10886-005-8404-1
Arn H, Louis F (1997) Mating Disruption in European Vineyards. In: Cardé R, Minks A (eds) Insect Pheromone Research. Springer US, pp 377–382. Doi:10.1007/978-1-4615-6371-6_33
Lord HL, Zhan WQ, Pawliszyn J (2010) Fundamentals and applications of needle trap devices: A critical review. Anal Chim Acta 677(1):3–18. doi:10.1016/j.aca.2010.06.020
Laaks J, Jochmann MA, Schmidt TC (2012) Solvent-free microextraction techniques in gas chromatography. Anal Bioanal Chem 402(2):565–571. doi:10.1007/s00216-011-5511-4
Weissbecker B, Holighaus G, Schutz S (2004) Gas chromatography with mass spectrometric and electroantennographic detection: analysis of wood odorants by direct coupling of insect olfaction and mass spectrometry. J Chromatogr A 1056(1–2):209–216. doi:10.1016/j.chroma.2004.06.120
Hübschmann H-J (2009) Handbook of GC/MS: fundamentals and applications, 2nd edn. Wiley-VCH, Weinheim, ompleted rev. and updated edn
Roelofs WL (1984) Electroantennogram Assays: Rapid and Convenient Screening Procedures for Pheromones. In: Hummel H, Miller T (eds) Techniques in Pheromone Research. Springer Series in Experimental Entomology. Springer, New York, pp 131–159
Koch UT, Doye E, Schumann K, Andrick U (2009) CIRCE – an addition to the toolbox for assessment / improvement of mating disruption. In: Tasin M, Witzgall P (eds) IOBC / WPRS - Working Group “Pheromones and other Semiochemicals in Integrated Production” Lund (Sweden), September 2007. International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palearctic Regional Section, pp 17–24
Currie LA, Svehla G (1994) Nomenclature for the Presentation of Results of Chemical-Analysis. Pure Appl Chem 66(3):595–608. doi:10.1351/pac199466030595
Tasin M, Anfora G, Ioriatti C, Carlin S, De Cristofaro A, Schmidt S, Bengtsson M, Versini G, Witzgall P (2005) Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J Chem Ecol 31(1):77–87. doi:10.1007/s10886-005-0975-3
Von Arx M, Schmidt-Busser D, Guerin PM (2011) Host plant volatiles induce oriented flight behaviour in male European grapevine moths, Lobesia botrana. J Insect Physiol 57(10):1323–1331. doi:10.1016/j.jinsphys.2011.06.010
Sauer AE, Koch UT (1989) Are filter papers reliable stimulating devices in insect olfactory investigations? In: Elsner N, Singer W (eds) Dynamics and Plasticity in Neuronal Systems: Proceedings of the 17th Göttingen Neurobiology Conference. Thieme, Stuttgart, p 78
Karg G, Suckling DM (1997) Polyethylene dispensers generate large-scale temporal fluctuations in pheromone concentration. Environ Entomol 26(4):896–905
Karg G, Suckling DM (2005) Applied Aspects of Insect Olfaction. In: Hansson BS (ed) Insect Olfaction. Springer, Berlin Heidelberg, pp 351–377
Myrick AJ, Park KC, Hetling JR, Baker TC (2009) Detection and Discrimination of Mixed Odor Strands in Overlapping Plumes Using an Insect-Antenna-Based Chemosensor System. J Chem Ecol 35(1):118–130. doi:10.1007/s10886-008-9582-4
Myrick AJ, Baker TC (2011) Locating a compact odor source using a four-channel insect electroantennogram sensor. Bioinspir Biomim 6 (1). doi:10.1088/1748-3182/6/1/016002
Carde RT (1990) Principles of mating disruption. In: Ridgway RL, Silverstein RM, Inscoe NM (eds) Behavior-Modifying Chemicals for Pest Management: Applications of Pheromones and Other Attractants. Marcel Dekker, New York, pp 42–71
Sanders CJ (1997) Mechanisms of Mating Disruption in Moths. In: Cardé R, Minks A (eds) Insect Pheromone Research. Chapman & Hall, New York, pp 333–346. doi:10.1007/978-1-4615-6371-6_29
Walgraeve C, Demeestere K, Dewulf J, Van Huffel K, Van Langenhove H (2011) Uptake rate behavior of tube-type passive samplers for volatile organic compounds under controlled atmospheric conditions. Atmos Environ 45(32):5872–5879. doi:10.1016/j.atmosenv.2011.06.069
Barnkob LL, Petersen JH (2013) Effect of relative humidity on the migration of benzophenone from paperboard into the food simulant Tenax (R) and modelling hereof. Food Addit Contam A 30(2):395–402. doi:10.1080/19440049.2012.741717
Wehrenfennig C, Schott M, Gasch T, Sauerwald T, Düring RA, Vilcinskas A, Kohl CD (2012) Laboratory characterization of metal-oxide sensors intended for in situ analyses of pheromones - SOMMSA approach. Phys Status Solidi A 209(5):935–939. doi:10.1002/pssa.201100586, Applications and Materials Science
Acknowledgments
The authors acknowledge project funding provided by the Hessian Ministry of Science and Art via the LOEWE research focus “AmbiProbe” which includes a generous grant to AV and RD. We thank Dr. Michael Breuer for support with L. botrana and discussions, Prof. Dr. Reineke for providing measurement sites in Geisenheim, and Stephan Fiedler for help with field measurements. The authors also thank Dr. Richard M. Twyman for editing the manuscript and Prof. Dr. Brückner for his valuable comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 79.1 kb)
Rights and permissions
About this article
Cite this article
Schott, M., Wehrenfennig, C., Gasch, T. et al. A portable gas chromatograph with simultaneous detection by mass spectrometry and electroantennography for the highly sensitive in situ measurement of volatiles. Anal Bioanal Chem 405, 7457–7467 (2013). https://doi.org/10.1007/s00216-013-7196-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7196-3