Abstract
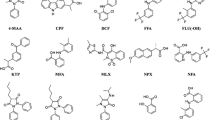
The main difficulties in analysing non-steroidal anti-inflammatory drugs (NSAIDs) in food and biological samples are due to the tight non-covalent interactions established with matrix proteins and the amount of occurring fatty material. The present paper describes an effective extraction procedure able to isolate fifteen NSAIDs (acetaminophen, salicylic acid, ibuprofen, diclofenac, flunixin and its metabolite 5-hydroxy-flunixin, nimesulide, phenylbutazone, meclofenamic acid, tolfenamic acid, meloxicam, carprofen, ketoprofen, naproxen and etodolac) from bovine milk and muscle tissue through two succeeding steps: (a) deproteinisation/extraction with organic solvent, essential to lower the medium dielectric constant and, therefore, to release the analytes from matrix; (b) SPE clean-up on OASIS cartridges. Lipids were easily removed during low-temperature centrifugations. The advantages of the developed procedure pertain to the efficient removal of the fat substances (very low matrix effect and high recovery yields) and its versatility, since it can be applied both to milk and muscle with few adjustments due to the diversity of the two matrices. Ion-pairing reversed-phase chromatography combined with the negative electrospray detection was able to achieve low detection capabilities (CCβs) for all analytes and, in particular, for diclofenac whose Maximum Residue Limit (MRL) in milk is 0.1 μg kg−1. The methods were validated according to the guidelines of the Commission Decision 2002/657/EC and then applied for a small monitoring study. A number of samples showed traces of salicylic acid (SA), but its occurrence was not ascribed to a misuse of drugs (aspirin, salicylic acid) since SA, accumulating in plants in response to a pathogen attack, may be introduced into the food chain.

Figurative representation of NSAIDs contamination in milk




Similar content being viewed by others
References
Thompson L (2005) Anti inflammatory Agents. In: Khan CM (ed) The Merck Veterinary Manual, 9th edn, Whitehouse Station: Merck & Co. Inc (http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/191605.htm (Accessed 12 March 2012)
Adams HR (2001) Veterinary pharmacology and therapeutics, 8th edn. Blackwell Publishing, Oxford
Hardman JG, Limbird LE (2001) Goodman and Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill Medical Publishing Division, New York
Smith GW, Davis JL, Tell LA, Webb AI, Riviere JE (2008) Extralabel use of nonsteroidal anti-inflammatory drugs in cattle. J Am Vet Med Assoc 232(5):697–701
Union European (1990) EU Council Regulation 2377/90/EEC of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off J Eur Commun L224:1–8
European Union (2010) Commission Regulation (EU) No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union L15:1–72
European Union (2007) CRL Guidance paper http://www.bvl.bund.de/SharedDocs/Downloads/09_Untersuchungen/EURL_Empfehlungen_Konzentrationsauswahl_Methodenvalierungen.pdf?__blob=publicationFile&v=2 (Accessed 12 May 2012)
Gentili A (2007) Determination of non-steroidal anti-inflammatory drugs in environmental samples by chromatographic and electrophoretic techniques. Anal Bioanal Chem 387:1185–1202
Farré M, Petrovic M, Barceló D (2007) Recently developed GC/MS and LC/MS methods for determining NSAIDs in water samples. Anal Bioanal Chem 387:1203–1214
Gentili A (2007) LC-MS methods for analyzing anti-inflammatory drugs in animal food products. TrAC, Trends Anal Chem 26:595–608
Daeseleire E, Mortier L, De Ruyck H, Geerts N (2003) Determination of flunixin and ketoprofen in milk by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 488:25–34
Stolker AAM, Rutgers P, Oosterink E, Lasaroms JJP, Peters RJB, van Rhijn JA, Nielen MWF (2008) Comprehensive screening and quantification of veterinary drugs in milk using UPLC-ToF-MS. Anal Bioanal Chem 391:2309–2322
Van Hoof N, De Wasch K, Poelmans S, Noppe H, De Brabander H (2004) Multi-residue liquid chromatography/tandem mass spectrometry method for the detection of non-steroidal anti-inflammatory drugs in bovine muscle: optimisation of ion trap parameters. Rapid Commun Mass Spectrom 18:2823–2829
Igualada C, Moragues F, Pitarch J (2007) Rapid method for the determination of non-steroidal anti-inflammatory drugs in animal tissue by liquid chromatography–mass spectrometry with ion-trap detector. Anal Chim Acta 586:432–439
Gallo P, Fabbrocino S, Vinci F, Fiori M, Danese V, Serpe L (2008) Confirmatory identification of sixteen non-steroidal anti-inflammatory drug residues in raw milk by liquid chromatography coupled with ion trap mass spectrometry. Rapid Commun Mass Spectrom 22:841–854
Malone EM, Dowling G, Elliott CT, Kennedy DG, Regan L (2009) Development of a rapid, multi-class method for the confirmatory analysis of anti-inflammatory drugs in bovine milk using liquid chromatography tandem mass spectrometry. J Chromatogr A 1216:8132–8140
Dowling G, Gallo P, Malone E, Regan L (2009) Rapid confirmatory analysis of non-steroidal anti-inflammatory drugs in bovine milk by rapid resolution liquid chromatography tandem mass spectrometry. J Chromatogr A 1216:8117–8131
Jedziniak P, Szprengier-Juszkiewicz T, Olejnik M, Żmudzki J (2010) Determination of non-steroidal anti-inflammatory drugs residues in animal muscles by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 672:85–92
Dowling G, Malone E, Harbison T, Martin S (2010) Analytical strategy for the determination of non-steroidal anti-inflammatory drugs in plasma and improved analytical strategy for the determination of authorised and non-authorised non-steroidal anti-inflammatory drugs in milk by LC–MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27:962–982
Dubreil-Cheéneau E, Pirotais Y, Bessiral M, Roudaut B, Verdon E (2011) Development and validation of a confirmatory method for the determination of 12 non steroidal anti-inflammatory drugs in milk using liquid chromatography-tandem mass spectrometry. J Chromatogr A 1218:6292–6301
Jedziniak P, Szprengier-Juszkiewicz T, Pietruk K, Śledzińska E, Żmudzki J (2012) Determination of non-steroidal anti-inflammatory drugs and their metabolites in milk by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. doi:10.1007/s00216-012-5860-7
Hu T, Peng T, Li X-J, Chen D-D, Dai H-H, Deng X-J, Yue Z-F, Wang G-M, Shen J-Z, Xia X, Ding S-Y, Zhou Y-N, Zhu A-L, Jiang H-Y (2012) Simultaneous determination of thirty non-steroidal anti-inflammatory drug residues in swine muscle by ultra-high-performance liquid chromatography with tandem mass spectrometry. J Chromatogr A 1219:104–113
Union European (2002) Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun L221:8–36
Document SANCO/2004/2726-rev 4 (2008) Guidelines for the implementation of Decision 2002/657/EC, http://ec.europa.eu/food/food/chemicalsafety/residueas/cons_2004-2726rev4_en.pdf (Accessed 12 March 2012)
Choi BK, Gusev AI, Hercules DM (1999) Postcolumn Introduction of an Internal Standard for Quantitative LC-MS Analysis. Anal Chem 71:4107–4110
Boyd RK (1993) Quantitative trace analysis by combined chromatography and mass spectrometry using external and internal standards. Rapid Commun Mass Spectrom 7:257–271
Dutta SK, Basu SK, Sen KK (2005) Physico-chemical aspects of protein binding of nimesulide. Indian J Pharmaceut Sci 67:243–246
Mączka P, Komsta L, Skibinski R, Gumieniczek A (2010) Theoretical studies on keto-enol tautomerism, gas phase acidity and spectral properties of phenylbutazone. Ann Univ Mariae Curie-Sklodowska 23:29–41
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Acknowledgements
The authors would like to thank Dr. Bruno Neri for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 133 kb)
Rights and permissions
About this article
Cite this article
Gentili, A., Caretti, F., Bellante, S. et al. Development and validation of two multiresidue liquid chromatography tandem mass spectrometry methods based on a versatile extraction procedure for isolating non-steroidal anti-inflammatory drugs from bovine milk and muscle tissue. Anal Bioanal Chem 404, 1375–1388 (2012). https://doi.org/10.1007/s00216-012-6231-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6231-0