Abstract
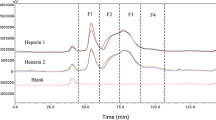
Protamine sulphate is an effective inhibitor of heparin and is used clinically to neutralise both low molecular weight heparins (LMWH) and unfractionated heparin (UFH). However, protamine sulphate does not fully counter the anti-Xa effect of LMWH, even in excess (>40 μg to 1 IU/ml). To investigate the molecular basis for this observation, the residual potencies in the presence and absence of plasma as well as the molecular weight profiles of commercial LMWH neutralised with increasing amounts of protamine were measured. Materials over 5000 Da are preferentially neutralised by protamine. To further investigate this molecular weight dependence, monodisperse oligosaccharides were prepared from three commercial LMWHs. The specific anti-Xa activity for the fractions increased with molecular weight, and was found to vary between the three preparations for oligosaccharides of the same molecular weight. Our results indicate that protamine sulphate neutralisation is largely dependent on molecular weight, leading to the implication that LMWHs containing a larger proportion of small oligosaccharides will not be as effectively neutralised. Protamine sulphate neutralisation of any given LMWH is also affected by the specific anticoagulant activities of its low molecular weight components, which varies between LMWH products, presumably with the method of manufacture.





Similar content being viewed by others
References
Yesook K, Rose CA (1995) Precipitation of insulinotropin in the presence of protamine: effect of phenol and zinc on the isophane ratio and the insulinotropin concentration in the supernatant. Pharm Res 12:1284–1288
Cundell RB, Jones GR, Murray D (1982) Polyelectrolytic complexes. 3. The interaction between heparin and protamine. Makromol Chem 183:849–861
Chang LC, Lee HF, YYang ZQ, Yang VC (2001) Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (I): preparation and characterization. AAPS Pharmsci 3:1–8
Andrassy K (1993) Low molecular weight heparin and haemodialysis: neutralization by protaminchloride. Blood Coagul Fibrinolysis 4:S39–S43
Leu JG, Chiang SS, Lin SM, Pai JK, Jiang WW (2000) Low molecular weight heparin in hemodialysis patients with a bleeding tendency. Nephron 86:499–501
Joannidis M, Kountchev J, Rauchenzauner M, Schusterschitz N, Ulmer H, Mayr A et al (2007) Enoxaparin vs. unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med 33:1571–1579
Al-Arrayed S, Seshadri R (2002) Use of low molecular weight heparin for hemodialysis: a short-term study. Saudi J Kidney Dis Transpl 13:146–150
Hirsh J, Raschke R (2004) Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:188S–203S
Makris M, Hough RE, Kitchen S (2000) Poor reversal of low molecular weight heparin by protamine. Br J Haematol 108:884–885
Holst J, Lindblad B, Bergqvist D, Garre K, Nielsen H, Hedner U et al (1994) Protamine neutralization of intravenous and subcutaneous low-molecular-weight heparin (tinzaparin, logiparin). An experimental investigation in healthy volunteers. Blood Coagul Fibrinolysis 5:795–803
Racanelli A, Hoppensteadt DA, Fareed J (1989) In vitro protamine neutralization profiles of heparins differing in source and molecular weight. Semin Thromb Hemost 15:386–389
Ramamurthy N, Baliga N, Wakefield TW, Andrews PC, Yang VC, Meyerhoff ME (1999) Determination of low-molecular-weight heparins and their binding to protamine and a protamine analog using polyion-sensitive membrane electrodes. Anal Biochem 266:116–124
Dawes J, Pepper DS (1982) A sensitive competitive binding assay for exogenous and endogenous heparins. Thromb Res 27:387–396
Crowther MA, Berry LR, Monagle PT, Chan AK (2002) Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br J Haematol 116:178–186
Kristensen HI, Tromborg EM, Nielsen JR, Nielsen JI, Johansen KB, Ostergaard PB (1991) Development and validation of a size exclusion chromatography method for determination of molecular masses and molecular mass distribution in low molecular weight heparin. Thromb Res 64:131–141
Mulloy B, Gee C, Wheeler SF, Wait R, Gray E, Barrowcliffe TW (1997) Molecular weight measurements of low molecular weight heparins by gel permeation chromatography. Thromb Haemost 77:668–674
Teien AN, Lie M, Abildgaard U (1976) Assay of heparin in plasma using a chromogenic substrate for activated factor X. Thromb Res 8:413–416
EDQM (2010) Heparins, low-molecular-mass (01/2008:0828). In: European pharmacopoeia. European Directorate for the Quality of Medicines and HealthCare (EDQM), Strasbourg
EDQM (2010) Assay of heparin (01/2008:20705). In: European pharmacopoeia. European Directorate for the Quality of Medicines and HealthCare (EDQM), Strasbourg
EDQM (2010) Conductivity (01/2008:20238). In: European pharmacopoeia. European Directorate for the Quality of Medicines and HealthCare (EDQM), Strasbourg
Beguin S, Choay J, Hemker HC (1989) The action of a synthetic pentasaccharide on thrombin generation in whole plasma. Thromb Haemost 61:397–401
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Heparin Characterization with Guest Editor Cynthia K. Larive.
Rights and permissions
About this article
Cite this article
Schroeder, M., Hogwood, J., Gray, E. et al. Protamine neutralisation of low molecular weight heparins and their oligosaccharide components. Anal Bioanal Chem 399, 763–771 (2011). https://doi.org/10.1007/s00216-010-4220-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4220-8