Abstract.
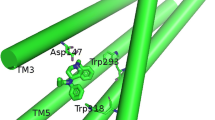
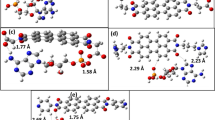
Ab initio self-consistent-field second-order Møller–Plesset perturbation theory computations including basis set superposition error and zero-point vibrational energy corrections have been performed on the complexation of benzene with the polar head of acetylcholine (ACh). The ACh–benzene complex is about 0.5 kcal/mol less stable than the corresponding tetramethylammonium (TMA)–benzene complex, with a structure a little distorted with respect to the latter. The electronic structure of ACh is little modified by the ligand. Overall, the replacement of one methyl group of TMA by the acetyl tail of ACh does not affect strongly the complexation to benzene, as far as the main interaction is concerned.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 1 April 1999 / Accepted: 19 October 1999 / Published online: 14 March 2000
Rights and permissions
About this article
Cite this article
Berthier, G., Savinelli, R. & Pullman, A. Cation π interaction between acetylcholine and the benzene ring. Theor Chem Acc 104, 78–81 (2000). https://doi.org/10.1007/s002149900107
Issue Date:
DOI: https://doi.org/10.1007/s002149900107