Abstract
The anti (a) to syn (s) isomerization pathway of the deprotonated form of the dimer with two nickel(II) 15-membered octaazamacrocyclic units connected via a carbon–carbon (C–C) σ bond was investigated. For the initial anti (a) structure, a deprotonation of one of the bridging (sp3 hybridized) carbon atoms is suggested to allow for an a to s geometry twist. A 360° scan around the bridging C–C dihedral angle was performed first to find an intermediate geometry. Subsequently, the isomerization pathway was explored via individual steps using a series of mode redundant geometry optimizations (internal coordinates potential energy surface scans) and geometry relaxations leading to the s structure. The prominent geometries (intermediates) of the isomerization pathway are chosen and compared to the a and s structures, and geometry relaxations of the protonated forms of selected intermediates are considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Investigation of transition-metal complexes with redox non-innocent ligands has been the subject of great research interest over the last few decades [1,2,3]. A detailed understanding of the electronic structures [4, 5] of these compounds and their use in studies of stoichiometric reactivity [6,7,8,9,10,11] and catalytic transformations [12,13,14] have been reported. Nowadays, the number of studies reporting the use of first-row transition-metal complexes with related ligands in catalytic reactions is increasing [15, 16]. Catalytic activity of nickel(II) complexes was reported in the oxidation of cyclohexane to cyclohexanol and cyclohexanone [17, 18], in the alcohol oxidation [15] and in the electrocatalytic reduction [19, 20] studies. Furthermore, the redox activity of nickel compounds was investigated in the perspective of the carbon dioxide reduction [21,22,23,24,25], water oxidation [26, 27] as well as photoredox catalytic C–C bond formation reactions [28,29,30]. Nickel(II) complexes with S-substituted isothiosemicarbazides (H2NNC(SR)NH2) and isothiosemicarbazones (R1R2C=NNC(SR)NH2) are known for their redox properties. The isothiocarbohydrazides (NH2NC(SR)NHNH2) and the isothiocarbohydrazones (R1R2C=NNC(SR)NHNH2) are closely related derivatives that exhibit redox non-innocent behavior [31, 32]. Recently, we have reported several new nickel(II) complexes with 14- and 15-membered octaazamacrocyclic ligands [33]. This study reported the structure and spectroscopic properties of 1e-oxidized and 1e-reduced species. Furthermore, the ability to catalyze microwave-assisted solvent-free oxidation of cyclohexane by tert-butyl hydroperoxide (TBHP) to an industrially significant mixture of cyclohexanol and cyclohexanone (that is, A/K oil) has also been reported [33].
The paramagnetic intermediates and final diamagnetic products generated after cathodic reduction of monomeric nickel(II) 15-membered octaazamacrocyclic complexes were studied by applying a collaboration of electrochemical and spectroelectrochemical techniques, including cyclic voltammetry, in situ EPR and EPR/UV–vis–NIR spectroelectrochemistry [34], as well as ex situ NMR and MS spectroelectrochemistry. Density functional theory (DFT) calculations were performed to further support the assignment of species generated after reduction [35]. In a follow-up work, the analysis of the relationship between the electronic structure of the 15-membered octaazamacrocyclic nickel complexes and their affinity for CO2 binding was addressed [33, 35, 36].
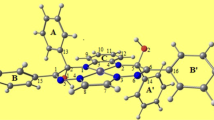
Although the number of mononuclear complexes with redox non-innocent ligands is large and increases permanently, reports on dinuclear metal complexes with this kind of ligands are still scarce [37,38,39]. Quite recently, Dobrov et al. [40] described the synthesis of diastereomeric dinickel(II) complexes (denoted as 4–6) with new bis(octaazamacrocyclic) ligands (denoted as 1–3), where Fig. 1 shows the anti (a) and syn (s) forms of 4 that will be further explored in this study. Geometric a and s isomers (enantiomers) were separated chromatographically, isolated, and characterized by analytical and spectroscopic techniques, as well as by single-crystal X-ray diffraction (SC-XRD) (4a and 4s, 5s, and 6a). The isomerization kinetics of 5 (the conversion of 5a to 5s) was investigated in chloroform by 1H NMR spectroscopy (it was monitored at five different temperatures from 20 °C to 50 °C), and the thermodynamics was compared with the theoretical DFT results [40]. The anti-syn isomerization (epimerization) of 5 was found to be a clean conversion from 5a to 5s, with the activation barrier of ΔH≠ = 114 ± 1 kJ mol–1 and the activation entropy of ΔS≠ = 13 ± 3 J K–1 mol–1 according to the Eyring equation [40]. It was shown that the half time of conversion from 5a (with a starting concentration of approximately x = 92%) to 5s was approximately 14 and 56 h at 50 and 40 °C, respectively (half times of conversions at 30 and 21 °C extrapolated from the kinetic model were 278 and 1111 h, respectively) as shown in Figure S5 in the Electronic Supplementary Information of Dobrov et al. [40]. Complex 4a was identified as the (R,S) or (S,R)-meso-isomer, where both form the same structure due to the presence of two identical monomeric units and an inversion center at the midpoint of the C15–C53 bridge (see Fig. 1). On the other hand, the complex 4s crystallized in a centrosymmetric triclinic space group \(P\overline{1 }\) (inversion center) as a racemic mixture of the (R,R)- and (S,S)-enantiomers [40]. It is worth noting that 4 is the only complex with crystal structures of both diastereomers 4a and 4s reported in the original reference [40].
In this paper, we extend the experimental study of Dobrov et al. [40] with a theoretical isomerization pathway consideration of an anti (a) dinuclear Ni(II) complex conformer, which is energetically less favored, to the energetically preferred syn (s) one. Therefore, the initial structure in this anti-syn isomerization pathway study is the anti (a) conformer. To allow the flexibility to undergo isomerization, we assume that one of the bridging sp3 hybridized carbons (C15) is deprotonated, see Fig. 1. For this initial structure of the anti conformer a, a scan of the bridging dihedral angle C54–C53–C15–C16 was performed in the range from 0 to 360° and a step size of 10°. Subsequently, the anti-syn isomerization pathway was explored using a potential energy surface (PES) scan with mode redundant (active/frozen) internal coordinate settings. Finally, selected C15 back-protonated intermediate structures were relaxed to report their anti-intermediate-syn preference.
1.1 Computational details
Standard all-electron (C, H, N, S, Ni) B3LYP/6-31G* [41,42,43,44,45,46,47,48] geometry optimizations in the preferred singlet spin state were performed using the Gaussian09 [49] program package. The stability of the optimized structures was confirmed by vibrational analysis (no imaginary frequencies). In addition, B3LYP calculations with the 6-311G** basis set [27,28,29,30,31,32,33,34] have been taken into account as well as the implicit integral equation formalism polarizable continuum model (IEFPCM) [50, 51] of CH2Cl2 to compare with the energetics of the in vacuo B3LYP/6-31G* calculations. Unless otherwise stated, in vacuo B3LYP/6-31G* calculations were reported. The potential energy surface scans were done with the opt(redundant) regime of Gaussian09, i.e., chosen internal coordinates are changed in a predefined sequence of steps, and kept frozen (constant) during the geometry optimization. Relative total SCF energies are considered in the potential energy surface scans, since a vast majority of these points are not minima on the potential energy surface with a frequency calculation (zero point correction) being biased due to nonzero gradients. Relative enthalpies and Gibbs free energies are presented for protonated and deprotonated structures that are minima on the potential energy surface. Atoms of internal coordinates which were scanned or frozen (see below) are labeled in Fig. 1. The IQmol package [52] has been used for the visualization of the obtained structures. The overlap of selected structures was visualized with the PyMOL package [53].
2 Results
According to the experimental findings, the anti (a) structure is energetically less favored than the syn (s) structure for complex 4. Therefore, the a isomer is chosen as the initial structure in this study of the anti-syn isomerization pathway. Additionally, to allow for the necessary flexibility, the C15 bridging sp3 hybridized carbon is deprotonated. The acidity of protons on these carbon atoms was found in monomeric species (the CH2 group of the monomer can donate a proton) [33]. In addition, the option of a tautomeric form of the dimeric species with a proton transfer from the carbon bridging atom to a nitrogen atom of the macrocycle has been considered. It was found that the total SCF energy difference between these species (123.6 kJ·mol–1) is already comparable to the experimentally estimated energy barrier (117 ± 1 kJ·mol–1) [40]. Thus, carbon C15 is considered as sp2 hybridized (lack of proton) in this study to allow exploration of the anti-syn isomerization pathway. The proton transfer B3LYP/6-31G* reaction total SCF energy, enthalpy and Gibbs free energy (aH + OH− → a + H2O) is –336, –300, and –306 kJ mol−1, respectively. The B3LYP/6-31G* total SCF energy, enthalpy, and Gibbs free energy differences between the deprotonated forms a and s obtained from the geometry optimization of crystal structure geometries are − 0.367, 0.974 and 0.110 kJ mol−1, respectively. In the case of protonated forms, the aH-sH B3LYP/6-31G* total SCF energy, enthalpy, and Gibbs free energy differences are − 5.413, − 4.826 and 0.958 kJ mol−1, respectively. In addition, when solvent effects become considered (IEFPCM model of CH2Cl2), the B3LYP/6-31G* total SCF energy, enthalpy, and Gibbs free energy differences between the deprotonated (protonated) forms a and s are − 0.929, 3.229, and − 0.297 (− 5.464, − 7.312, and − 6.073) kJ mol−1, respectively. Implicit IEFPCM model of CH2Cl2 B3LYP/6-311G** total SCF energy, enthalpy, and Gibbs free energy differences between the deprotonated (protonated) forms a and s are − 0.502, 0.029, and 0.000 (− 4.072, − 4.742, and − 0.004) kJ mol−1, respectively. Hence, the in vacuo B3LYP/6-31G* calculations lead to an acceptable agreement with a higher quality basis set and implicit solvent effects [54]. It needs to be also mentioned that the accuracy of DFT functionals, where B3LYP is being the part of most of these studies, regarding thermochemistry, is at a limit of 5 kJ mol−1, and often is reported to be worse than 15 kJ mol−1 [54,55,56]. Below, the labels a and s denote the deprotonated forms of 4, while the protonated forms are labeled as aH and sH.
2.1 Scan of the bridging dihedral angle
Prior to the actual anti-syn isomerization study, a relaxed scan of the bridging dihedral angle of C54–C53–C15–C16 atoms from 0° to 360° was performed for the initial deprotonated structure a, see in Fig. 2. The found minima and maxima on the PES are highlighted as a1–a8, their total B3LYP/6-31G* energies are compiled in Table S1. In the scan, we found a minimum (a2), which is to be related to the relaxation of one of the phenyl rings (C66; see Fig. 1) to an energetically preferred orientation. The structure a2 is below the initial structure by − 9.796 kJ mol−1 (where a = a1). After the full scan, the new relaxed structure a8 is 11.635 kJ mol−1 below the initial structure a. The difference in the structures a and a8 is shown in Fig. 4a, here we can see the perpendicular orientations of R2 phenyl rings on C54 (note that the a2 and a8 structures are readily the same, not shown). Furthermore, there are the maxima on the dihedral angle scan, a3, a5, and a7 which are 52.529 (64.164), 70.960 (82.595), 21.043 (32.678) kJ mol−1, respectively, above the initial structure a (a8). From the PES scan, it can be concluded that structure a8 can be considered the starting point of the anti-syn isomerization of the studied Ni macrocyclic dimer (let us emphasize again that we are considering the deprotonated form). According to the dihedral angle C54–C53–C15–C16 scan, the structure a7 will be considered as the first intermediate geometry of this anti-syn isomerization pathway study, according to the lower height of the barrier comparing to a3.
In addition, dihedral angle scans of C-SMe and phenyl groups are shown in Supplementary Materials (see Figs. S1 and S2). In the case of the scan of N46–C54–C66–C67 (phenyl group), the maximum on this PES is below 24.6 kJ mol−1, see Fig. S1. The total SCF energy difference between the first and last geometry of the scan is − 0.354 kJ mol−1. The found maxima of the scan of the dihedral angle N47-C55-S41-C58 (C-SMe group) are below 28.8 kJ mol−1 and 31.82 kJ mol−1, see Fig. S2.
2.2 Scan of the potential energy surface: the anti-syn isomerization pathway
As already mentioned, the obtained geometry a8 is the start for the PES exploitation to suggest an anti-syn isomerization pathway. The found isomerization pathway with the description of the individual steps/intermediates (a–g) is shown in Fig. 3, including the optimization details, i.e., active and frozen (PES scan, non-redundant) coordinates, or geometry relaxation (ordinary or constrained geometry optimization). The Cartesian coordinates of optimized geometries (a–s) are accounted for in Supplementary Materials (see Tables S3–S15). The differences (overlaps) of succeeding structures during the PES exploitation with the initial structure a8 are shown in Fig. 4, and the overlap of the selected geometries with the final syn (s) structure is compiled in Fig. 5, to show the additional changes in geometry (e.g., phenyl and C-SMe groups orientation).
Constrained potential energy surface scan. On the independent axis (x) is the anti-syn isomerisation pathway and on the dependent axis (y) is the total SCF energy difference in kJ mol–1 relative to a. Details of the isomerization pathway (scanned and frozen coordinates) are presented in the particular color
The difference in the backbone of structures a–a8 during the relaxation of the crystal structure like geometry a is due to the C66 phenyl ring reorientation, see Fig. 4a (a8 is the cyan reference structure). The difference of a8–a7 structures during the C54–C53–C15–C16 dihedral angle scan, from 135.8 to 185.8°, step = 10°, #steps = 5 is shown in Fig. 4b (a7 is the dark blue structure).
The geometry of structure a7 was then used for a further C53–C15–Ni1 angle PES scan keeping the Ni78-C53-C15 angle frozen, see orange points in Fig. 3. The overlap of structures a8-b3 is shown in Fig. 4c. The PES maximum of b2 is 135.0 (146.7) kJ mol–1, above the initial structure a (a8), see Table S1 and Fig. 3.
The overlap of the initial structures a8 and c1 (see gray structure in Fig. 4d) shows the difference due to the relaxation of the angle C53–C15–Ni1 which was frozen in b (orange points in Fig. 3). The C53–C15–Ni1 angle changes from 169° in b3 to 155° in c1. The energy difference between b3–c1 is –13.608 kJ·mol–1. Surprisingly, the energetically preferred structure for the deprotonated form is c1. The existence of the c1 minimum in the PES of the deprotonated forms is to be considered an important intermediate on the isomerization pathway. The protonated form of c1H is not energetically preferred compared to aH and sH, see Table 1 and in the next section devoted to geometry relaxations of the back-protonated forms.
The gray part (c1–d1) of the isomerization pathway was based on the scan of the Ni78-C53-Ni1 angle (see Fig. 3). The energy difference between c1 and d1 is –22.460 kJ·mol–1 (d1 is above c1, see Table S1, Fig. 3). In this part, the actual isomerization from anti (a) to syn (s) happens. The structure d1 (see the red structure in Figs. 4e and 5a) is by 0.844 (10.791) kJ mol–1 below the initial structure a (a8).
The red and yellow part of the isomerization pathway (see Fig. 3) continues with the C54–C53–C15–C16 dihedral angle PES scan from 112° to 72° (68° to 32°) for d1–d2 (d2–d3) structures, respectively, see Fig. 3. The PES maximum of the yellow part (not labeled) is 56.569 (68.203) kJ mol–1 above the initial structure a (a8). The overlaps of d2 (pink structure) with a8 and s are shown in Figs. 4f and 5b, respectively. After the d scan, the d3 geometry was used as the input for a geometry optimization/relaxation (see structure e in Fig. 3). The overlap of the structures d3-s is essentially the same as for e-s (green structure) shown in Fig. 5c, i.e., the major difference between d3 and the s isomer is the orientation of the C66 phenyl ring. The energy difference between structure a and e is 8.331 kJ mol–1. Thus, the next PES scan (e to f) was performed for the rotation of the C66 phenyl group of e (see Fig. 3).
The final geometry optimization was performed for the structure g (see the brown part in Fig. 3). The close overlap of g (brown structure) with the s isomer is shown in Fig. 5d. The structure g is –0.359 kJ mol–1 below the initial structure a (respectively, the structure g is 11.275 kJ mol–1 above a8) in total SCF energy. Note that s is by –0.367 kJ mol–1 below a, therefore, g should be considered energetically degenerate with s. Relative enthalpies and Gibbs free energies are provided for selected relaxed deprotonated structures in Table S2.
2.3 Stability and relaxation of the back-protonated intermediates
As a final step within this study, the relaxation of the back-protonated forms of selected intermediate structures was performed. A proton has been added to the carbon C15 atom being sp3sp3hybridized in the (back-)protonated forms.
The protonated form of a is denoted as aH. The intermediate structure b1H relaxes to the anti isomer. This structure defines the actual energy minimum of the protonated a forms. Relaxed intermediate structures of b3H and c1H are energetical and geometric equivalents of an anti isomer with a bridging dihedral angle C54–C53–C15–C16 of 76° (that is, an a6H perpendicular like structure of Fig. 2). The structure d1H is the anti to syn cross intermediate structure (the bridging dihedral angle C54–C53–C15–C16 is 78° and the angle Ni78–C53–Ni1 is 173°). The remaining structures (from d2H to gH) relaxed to the geometry of a syn isomer (with d2H being an a4H perpendicular like structure of Fig. 2). The protonated syn structure has two possible variants with respect to chirality of the bridging carbons. Actually, the original structures were found as the racemic mixtures of S,S and R,R and the isomerization pathway resulted in the R,R isomer. Furthermore, the difference in the preference of the 1[aH]0 and 1[sH]0 structures hints on the complexity of the PES structure with respect to the presence of several local minima, exploitation of which is beyond the scope of this work. Still, the activation barrier of the deprotonated forms is in agreement with the experimental kinetics reported for compound 5 in the original study. It can be assumed that the enforced (mode redundant) geometry PES scans lead to an overestimated energy barrier in Fig. 3, structure b2. Furthermore, deprotonation needs to occur, to activate the suggested isomerization pathway. Another option is the tautomerization of the proton to any of the nitrogens on the ligand macrocycles, as discussed in the original reference [40], to activate the mechanism of the carbon–carbon isomerization pathway. The found anti and syn pronated forms are to be qualified degenerate, and albeit the crystal structure relaxed geometries led to the thermodynamic preference of the syn structure, the degenerate situation is acceptable with respect to the accuracy of DFT calculations [54,55,56].
3 Conclusions
The anti-syn isomerization pathway of the deprotonated form of dinickel(II) complexes with C–C coupled 15-membered octaazamacrocyclic ligand was successfully investigated theoretically. The initial anti structure a8 and final structure s, were found a local minima in comparison with the found c1 structure. The actual isomerization from anti (a) to syn (s) is found to occur in the part c1–d1 of the followed pathway.
Finally, the relaxation of the back-protonated forms of selected intermediate structures of the isomerization pathway was performed. According to our results, structures b1H, b3H and c1H are relaxing back to the anti isomer. The structure d1Hd1His the anti to syn cross intermediate structure. While structures from d2H to gH relaxed to the geometry of the syn isomer. The protonated syn structure has two possible chiral variants, and the isomerization pathway followed herein led to the R,R enantiomer, note that both crystal types, i.e., (R,R) and (S,S), were found in the original study [40]. A further issue is the energy barrier height, which is found to be overestimated in comparison with the experimentally derived value. In this place, we need to accept that the guided PES scan was unable to find the optimal path when crossing from the b1 to the b3 intermediate. The found back-protonated structures are found to be energetically degenerate which is acceptable with respect to the accuracy of DFT calculations [54,55,56].
4 Supplementary Materials
Figures of dihedral angle scan of phenyl and C-SMe groups for the s structure; Table of total and relative SCF energies for selected deprotonated structures; Table of relative enthalpies and Gibbs free energies of selected relaxed deprotonated structures; and the Cartesian coordinates of selected geometries (a-s) are provided in a docx file.
References
Kaim W, Schwederski B (2010) Non-innocent ligands in bioinorganic chemistry—an overview. Coord Chem Rev 254:1580–1588
Kaim W (2012) The shrinking world of innocent ligands: conventionaland non‐conventional redox‐active ligands. Europ J Inorg Chem 3:343–348
Chirik PJ (2011) Preface: forum on redox-active ligands. Inorg Chem 50:9737–9740
Storr T, Wasinger EC, Pratt RC, Stack TDP (2007) The geometric and electronic structure of a one-electron-oxidized nickel(II) bis(salicylidene)diamine complex. Angew Chem 119:5290–5293. https://doi.org/10.1002/ange.200701194
Shimazaki Y, Yajima T, Tani F, Karasawa S, Fukui K, Naruta Y, Yamauchi O (2007) Syntheses and electronic structures of one-electron-oxidized group 10 metal(II)−(disalicylidene)diamine complexes (metal = Ni, Pd, Pt). J Am Chem Soc 129:2559–2568. https://doi.org/10.1021/ja067022r
Kaim W (2011) Manifestations of noninnocent ligand behavior. Inorg Chem 50:9752–9765
Sproules S, Wieghardt K (2010) o-Dithiolene and o-aminothiolate chemistry of iron: synthesis, structure and reactivity. Coord Chem Rev 254:1358–1382
Ray K, Petrenko T, Wieghardt K, Neese F (2007) Joint spectroscopic and theoretical investigations of transition metal complexes involving non-innocent ligands. Dalton Trans 16:1552–1566
Haas K, Ponikwar W, Nöth H, Beck W (1998) Facile synthesis of cyclic tetrapeptides from nonactivated peptide esters on metal centers. Angew Chem Int Ed 37:1086–1089. https://doi.org/10.1002/(SICI)1521-3773(19980504)37:8%3c1086::AID-ANIE1086%3e3.0.CO;2-V
Haas K, Dialer H, Piotrowski H, Schapp J, Beck W (2002) Selective α-carbon hydroxylation of glycine in nickel(II)–cyclotetrapeptide complexes by oxygen. Angew Chem Int Ed 41:1879–1881. https://doi.org/10.1002/1521-3773(20020603)41:11%3c1879::AID-ANIE1879%3e3.0.CO;2-K
Lu CC, Weyhermüller T, Bill E, Wieghardt K (2009) Accessing the different redox states of α-iminopyridines within cobalt complexes. Inorg Chem 48:6055–6064
Chirik PJ, Wieghardt K (2010) Radical ligands confer nobility on base-metal catalysts. Science 327:794–795. https://doi.org/10.1126/science.1183281
Lyaskovskyy V, de Bruin B (2012) Redox non-innocent ligands: versatile new tools to control catalytic reactions. ACS Catal 2:270–279. https://doi.org/10.1021/cs200660v
Storr T, Mukherjee R (2018) Preface for the forum on applications of metal complexes with ligand-centered radicals. Inorg Chem 57:9577–9579. https://doi.org/10.1021/acs.inorgchem.8b02171
Sikari R, Sinha S, Jash U, Das S, Brandao P, de Bruin B, Paul ND (2016) Deprotonation induced ligand oxidation in a NiII complex of a redox noninnocent N1-(2-aminophenyl) benzene-1, 2-diamine and its use in catalytic alcohol oxidation. Inorg Chem 55:6114–6123
Najafian A, Cundari TR (2017) Methane C–H activation via 3d metal methoxide complexes with potentially redox-noninnocent pincer ligands: a density functional theory study. Inorg Chem 56:12282–12290
Sankaralingam M, Balamurugan M, Palaniandavar M, Vadivelu P, Suresh CH (2014) Nickel (II) complexes of pentadentate N5 ligands as catalysts for alkane hydroxylation by using m-CPBA as oxidant: a combined experimental and computational study. Chem A Eur J 20:11346–11361
Bilyachenko AN, Yalymov AI, Shul’pina LS, Mandelli D, Korlyukov AA, Vologzhanina AV, Es’kova MA, Shubina ES, Levitsky MM, Shul’pin GB (2016) Novel cage-like hexanuclear nickel (II) silsesquioxane. Synthesis, structure, and catalytic activity in oxidations with peroxides. Molecules 21:665
Taniguchi I, Nakashima N, Matsushita K, Yasukouchi K (1987) Electrocatalytic reduction of nitrate and nitrite to hydroxylamine and ammonia using metal cyclams. J Electroanal Chem Interfac Electrochem 224:199–209
Efros LL, Thorp HH, Brudvig GW, Crabtree RH (1992) Towards a functional model of hydrogenase: electrocatalytic reduction of protons to dihydrogen by a nickel macrocyclic complex. Inorg Chem 31:1722–1724
Fisher BJ, Eisenberg R (1980) Electrocatalytic reduction of carbon dioxide by using macrocycles of nickel and cobalt. J Am Chem Soc 102:7361–7363
de Alwis C, Crayston JA, Cromie T, Eisenblätter T, Hay RW, Lampeka YD, Tsymbal LV (2000) Cyclic voltammetry study of the electrocatalysis of carbon dioxide reduction by bis (polyazamacrocyclic) nickel complexes. Electrochim Acta 45:2061–2074
Yamazaki Y, Takeda H, Ishitani O (2015) Photocatalytic reduction of CO2 using metal complexes. J Photochem Photobiol C Photochem Reviews 25:106–137
Gerschel P, Warm K, Farquhar ER, Englert U, Reback ML, Siegmund D, Ray K, Apfel U-P (2019) Sulfur substitution in a Ni(cyclam) derivative results in lower overpotential for CO2 reduction and enhanced proton reduction. Dalton Trans 48:5923–5932. https://doi.org/10.1039/C8DT04740E
Neri G, Forster M, Walsh JJ, Robertson CM, Whittles TJ, Farràs P, Cowan AJ (2016) Photochemical CO2 reduction in water using a co-immobilised nickel catalyst and a visible light sensitiser. Chem Commun 52:14200–14203
Shen J, Wang M, He T, Jiang J, Hu M (2018) Influence of the backbone of N 5-pentadentate ligands on the catalytic performance of Ni (II) complexes for electrochemical water oxidation in neutral aqueous solutions. Chem Commun 54:9019–9022
Han Y, Wu Y, Lai W, Cao R (2015) Electrocatalytic water oxidation by a water-soluble nickel porphyrin complex at neutral pH with low overpotential. Inorg Chem 54:5604–5613
Nakajima K, Nojima S, Nishibayashi Y (2016) Nickel-and photoredox-catalyzed cross-coupling reactions of aryl halides with 4-Alkyl-1, 4-dihydropyridines as formal nucleophilic alkylation reagents. Angew Chem 128:14312–14316
Lima F, Kabeshov MA, Tran DN, Battilocchio C, Sedelmeier J, Sedelmeier G, Schenkel B, Ley SV (2016) Visible light activation of boronic esters enables efficient photoredox C (sp2)–C (sp3) cross-couplings in flow. Angew Chem 128:14291–14295
Johnston CP, Smith RT, Allmendinger S, MacMillan DWC (2016) Metallaphotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides. Nature 536:322–325
Arion VB (2019) Coordination chemistry of S-substituted isothiosemicarbazides and isothiosemicarbazones. Coord Chem Rev 387:348–397
Gerbeleu NV, Arion VB, Burgess J (1999) Template synthesis of macrocyclic compounds. Wiley-VCH, Weinheim
Dobrov A, Darvasiová D, Zalibera M, Bučinský L, Puškárová I, Rapta P, Shova S, Dumitrescu D, Martins LMDRS, Pombeiro AJL, Arion VB (2019) Nickel(II) complexes with redox noninnocent octaazamacrocycles as catalysts in oxidation reactions. Inorg Chem 58:11133–11145. https://doi.org/10.1021/acs.inorgchem.9b01700
Rapta P, Dmitrieva E, Popov AA, Dunsch L (2015) In situ spectroelectrochemistry of Organic Compounds. Organic electrochemistry, 5th edn. CRC Press, Boca Raton, FL, USA, pp 169–190
Darvasiová D, Šoral M, Puškárová I, Dvoranová D, Vénosová B, Bučinský L, Zalibera M, Dujnič V, Dobrov A, Schwalbe M, Arion VB, Rapta P (2019) Spectroelectrochemical, photochemical and theoretical study of octaazamacrocyclic nickel(II) complexes exhibiting unusual solvent-dependent deprotonation of methylene group. Electrochim Acta 326:135006. https://doi.org/10.1016/j.electacta.2019.135006
Venosova B, Jelemenska I, Kozisek J, Rapta P, Zalibera M, Novotny M, Arion VB, Bucinsky L (2021) Ni oxidation state and ligand saturation impact on the capability of octaazamacrocyclic complexes to bind and reduce CO2. Molecules. https://doi.org/10.3390/molecules26144139
van Leest NP, Tepaske MA, Oudsen J-PH, Venderbosch B, Rietdijk NR, Siegler MA, Tromp M, van der Vlugt JI, de Bruin B (2020) Ligand redox noninnocence in [CoIII(TAML)]0/– complexes affects nitrene formation. J Am Chem Soc 142:552–563. https://doi.org/10.1021/jacs.9b11715
Goswami M, Lyaskovskyy V, Domingos SR, Buma WJ, Woutersen S, Troeppner O, Ivanović-Burmazović I, Lu H, Cui X, Zhang XP, Reijerse EJ, DeBeer S, van Schooneveld MM, Pfaff FF, Ray K, de Bruin B (2015) Characterization of porphyrin-Co(III)-‘nitrene radical’ species relevant in catalytic nitrene transfer reactions. J Am Chem Soc 137:5468–5479. https://doi.org/10.1021/jacs.5b01197
Luca OR, Crabtree RH (2013) Redox-active ligands in catalysis. Chem Soc Rev 42:1440–1459. https://doi.org/10.1039/C2CS35228A
Dobrov A, Darvasiova D, Zalibera M, Bucinsky L, Jelemenska I, Rapta P, Shova S, Dumitrescu DG, Andrade MA, Martins L, Pombeiro AJL, Arion VB (2022) Diastereomeric dinickel(II) complexes with non-innocent bis(octaazamacrocyclic) ligands: isomerization, spectroelectrochemistry, DFT calculations and use in catalytic oxidation of cyclohexane. Dalton Trans 51:5151–5167. https://doi.org/10.1039/d2dt00154c
Wachters AJH (1970) Gaussian basis set for molecular wavefunctions containing third-row atoms. J Chem Phys 52:1033–1036. https://doi.org/10.1063/1.1673095
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z =11–18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654. https://doi.org/10.1063/1.438955
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211. https://doi.org/10.1139/p80-159
BeckE AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A (Coll Park) 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Becke AD (1993) Becke’s three parameter hybrid method using the LYP correlation functional. J Chem Phys 98:5648–5652
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz J V, Cioslowski J, Fox DJ (2014) Gaussian 09 Revision D.01
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335. https://doi.org/10.1016/0009-2614(96)00349-1
Cossi M, Barone V, Mennucci B, Tomasi J (1998) Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem Phys Lett 286:253–260. https://doi.org/10.1016/S0009-2614(98)00106-7
Gilbert A (2018) IQmol, Version v2.10
Schrödinger LLC (2015) The PyMOL molecular graphics system, Version 1.8
Sousa SF, Fernandes PA, Ramos MJ (2007) General performance of density functionals. J Phys Chem A 111:10439–10452. https://doi.org/10.1021/jp0734474
Petersson GA, Malick DK, Wilson WG, Ochterski JW, Montgomery JA, Frisch MJ (1998) Calibration and comparison of the Gaussian-2, complete basis set, and density functional methods for computational thermochemistry. J Chem Phys 109:10570–10579
Bogojeski M, Vogt-Maranto L, Tuckerman ME, Müller K-R, Burke K (2020) Quantum chemical accuracy from density functional approximations via machine learning. Nat Commun 11:5223. https://doi.org/10.1038/s41467-020-19093-1
Acknowledgements
This work was supported by the Science and Technology Assistance Agency (Contract Nos. APVV-19-0024, APVV-20-0213 and DS-FR-19-0035) and VEGA (Contract Nos. 1/0139/20, 1/0078/21 and 1/0175/23) We thank the HPC centre at the Slovak University of Technology in Bratislava, which is a part of the Slovak Infrastructure of High Performance Computing (SIVVP Project, ITMS code 26230120002, funded by the European Region Development Funds), for computing facilities. This article was written thanks to the generous support under the Operational Program Integrated Infrastructure for the project: "Support of research activities of Excellence laboratories STU in Bratislava", Project No. 313021BXZ1, co-financed by the European Regional Development Fund. VBA thanks the Austrian Science Fund (FWF) for the financial support of this work via the Project I4729. The open access has been provided by the Slovakia CVTI Consortium Transformative Agreement with Springer Nature.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Contributions
IJ contributed to the conceptualization, methodology, formal analysis, data Curation, investigation, writing—original draft, and visualization. MZ was involved in the conceptualization, writing—review and editing, and funding acquisition. PR contributed to the resources, writing—review and editing, and funding acquisition. AAD assisted in the conceptualization, resources, writing—review and editing. VBA was involved in the conceptualization, methodology, resources, writing—review and editing, and funding acquisition. LB performed the conceptualization, methodology, writing—original draft, writing—review and editing, supervision, project administration, software, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jelemenska, I., Zalibera, M., Rapta, P. et al. Isomerization pathway of a C–C sigma bond in a bis(octaazamacrocycle)dinickel(II) complex activated by deprotonation: a DFT study. Theor Chem Acc 143, 26 (2024). https://doi.org/10.1007/s00214-024-03100-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03100-5