Abstract
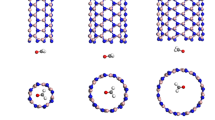
The boron nitrides as the excellent sensors are used to detect certain harmful gases. The diameter of the boron nitrides is an important structural parameter to adjust the adsorption capacity. The structures, stabilities and electronic attributes of the NO2BmNm and NO2@BmNm (m = 48, 72 and 96) clusters have been investigated via first-principles. The bond angle ∠O–N–O = 134° of free NO2 molecules becomes slightly narrow (129.171°, 128.911° and 128.593°; 124.050°, 123.578° and 124.237°) of the NO2BmNm and NO2@BmNm clusters. The NO2 molecules prefer to embed in larger diameter BmNm (m = 72 and 96) clusters by the calculated binding energies per atom and HOMO–LUMO gaps. The charge amounts of the O2 fragments of the NO2BmNm clusters are almost the same while those of O2 fragments of the NO2@BmNm clusters obviously reduce. The internal charges of the O atoms of the NO2BmNm and NO2@BmNm (m = 48, 72 and 96) clusters transfer from the s to d orbitals.







Similar content being viewed by others
References
Farmanzadeh D, Rezainejad H (2016) Appl Surf Sci 364:862
Fan G, Zhu S, Li X, Ni K, Xu H (2017) Comput Theor Chem 1115:208
He W, Li Z, Yang J, Hou JG (2008) J Chem Phys 128:164701
Peyghan AA, Soltani A, Pahlevani AA, Kanani Y, Khajeh S (2013) Appl Surf Sci 270:25
Xie Y, Huo Y-P, Zhang J-M (2012) Appl Surf Sci 258:6391
Esrafili MD (2013) Struct Chem 24:1207
Deng Z-Y, Zhang J-M (2016) Can J Phys 94:1071
Deng Z-Y, Zhang J-M, Xu K-W (2016) Phys E 76:47
Xiao M, Li X, Du B, Han T, Li Z, Li J, Xing Y (2019) Appl Surf Sci 491:698
Soltani A, Baei MT, Lemeski ET, Kaveh S, Balakheyli H (2015) J Phys Chem Solids 86:57
Soltani A, Ahmadian N, Kanani Y, Dehnokhalaji A, Mighani H (2012) Appl Surf Sci 258:9536
Anota EC, Cocoletzi GH (2014) Phys E 56:134
Anota EC, Cocoletzi GH, Sánchez Ramírez JF, Hernández AB (2014) Struct Chem 25:895
He W, Li Z, Yang J, Hou JG (2008) J Chem Phys 129:024710
Karmodak N, Jemmis ED (2016) Chem Asian J 11:3350
Beheshtian J, Kamfiroozi M, Bagheri Z, Peyghan AA (2012) Chin J Chem Phys 25:60
Bawa FH (2010) J Chem Soc Pak 32:319
Delley B (1990) J Chem Phys 92:508
Delley B (2000) J Chem Phys 113:7756
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Mananghaya MR (2018) Int J Hydrog Energ 43:10368
Tereshchuk P, Silva JLFD (2012) Phys Rev B 85:195461
Zhao Z, Li Z, Wang Q, Wang D, Wu C, Zhou Z (2016) Comput Theor Chem 1095:9
Li Z, Zhou Z, Wang H, Li S, Zhao Z (2016) J Cryst Growth 449:22
Zhao Z, Zhang T, Wu J, Li Z (2022) Eur Phys J Plus 137:1027
Li Z, Zhao Z (2018) Phase Transit 91:426
Li Z, Zhao Z, Wang Q, Shi T (2019) Phase Transit 92:360
Li Z, Zhao Z, Wang Q, Shi T (2019) Phase Transit 92:537
Li Z, Zhao Z (2017) J Mater Sci 52:3301
Kootenaei AS, Ansari G (2016) Phys Lett A 380:2664
Wang H (2010) Chin J Chem 28:1897
Zhao Z, Li Z, Shen X (2021) Mater Chem Phys 260:124098
Matxain JM, Eriksson LA, Formoso E, Piris M, Ugalde JM (2007) J Phys Chem C 111:3560
Valentin CD, Wang F, Pacchioni G (2013) Top Catal 56:1404
Zhao YR, Xu YQ, Chen P, Yuan YQ, Qian Y, Li Q (2021) Results Phys 26:104341
Zhao YR, Bai TT, Jia LN, Xin W, Hu YH, Zheng XS (2019) J Phys Chem C 123:28561
Zhang XY, Zhao YR, Li HX, Cheng KG, Liu ZR, Liu ZP, He H (2023) Chin Phys B 32:066102
Acknowledgments
The authors would like to acknowledge the financial support from the National Natural Science Foundation, People’s Republic of China (Grant No.51634004) and top academic talent training program of Anshan Normal University (23kyxm0001).
Author information
Authors and Affiliations
Contributions
Zhi Li: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Jia-cong Li, Jia-hui Yin, Shu-qi Yang: Investigation, Writing - review & editing. Zhen Zhao: Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Li, Jc., Yin, Jh. et al. Structures, stabilities and electronic properties of nitrogen dioxide adsorbed and embedded boron nitride clusters with different diameters. Theor Chem Acc 142, 113 (2023). https://doi.org/10.1007/s00214-023-03058-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03058-w