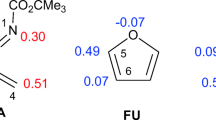
Abstract
Within the framework of the reaction force analysis, numerical data obtained from DFT calculations were used to characterize the mechanism of three Diels–Alder reactions involving three substituted furandione as dienophile, and a chiral anthracene, as diene. Then, the Marcus potential energy function and the activation strain model were used to rationalize the energetics of the reactions and to obtain physical insights on the nature of activation energies. It has been found that the activation processes are dominated by structural arrangements of reactants, basically due to the approach of the diene to the dienophile to start the reaction. Besides, the electronic activity taking place along the reaction coordinate have been analyzed through the reaction electronic flux. It has been found that the electronic activity that emerge more intensively within the transition-state region, is mainly due to electronic transfer effects, due to the breaking and forming π bonds. Although polarization effects are also present but to a lesser extent.





Similar content being viewed by others
References
Trivedi B (2013) Maleic anhydride
Nagaraja A, Jalageri MD, Puttaiahgowda YM, Raghava Reddy K, Raghu AV (2019) A review on various maleic anhydride antimicrobial polymers. J Microbiol Methods 163:105650
Shen Y, Qi R, Liu Q, Wang Y, Mao Y, Yu J (2008) Grafting of maleic anhydride onto polyethylene through a green chemistry approach. J Appl Polym Sci 110:2261–2266
Mahmoud E, Watson DA, Lobo RF (2014) Renewable production of phthalic anhydride from biomass-derived furan and maleic anhydride. Green Chem 16:167–175
Li X, Ho B, Zhang Y (2016) Selective aerobic oxidation of furfural to maleic anhydride with heterogeneous Mo-V-O catalysts. Green Chem 18:2976–2980
Sakata K, Fujimoto H (2016) origin of the endo selectivity in the diels-alder reaction between cyclopentadiene and maleic anhydride. European J Org Chem 2016:4275–4278
Goh YW, White JM (2009) Structure correlation study of some diels-alder cycloadducts of anthracene. Aust J Chem 62:419–424
Hernvann F, Rasore G, Declerck V, Aitken DJ (2014) Stereoselective intermolecular [2 + 2]-photocycloaddition reactions of maleic anhydride: stereocontrolled and regiocontrolled access to 1,2,3-trifunctionalized cyclobutanes. Org Biomol Chem 12:8212–8222
Domingo LR, Sáez JA (2009) Understanding the mechanism of polar Diels Alder reactions. Org Biomol Chem 7:3576–3583
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748
Yepes D, Murray JS, Pérez P, Domingo LR, Politzer P, Jaque P (2014) Complementarity of reaction force and electron localization function analyses of asynchronicity in bond formation in Diels-Alder reactions. Phys Chem Chem Phys 16:6726–6734
Corbett MS, Liu X, Sanyal A, Snyder JK (2003) Cycloadditions of chiral anthracenes: effect of the trifluoromethyl group. Tetrahedron Lett 44:931–935
Echegaray E, Toro-Labbé A (2008) Reaction electronic flux: a new concept to get insights into reaction mechanisms study of model symmetric nucleophilic substitutions. J Phys Chem A 112:11801–11807
Cerón ML, Echegaray E, Gutiérrez-Oliva S, Herrera B, Toro-Labbé A (2011) The reaction electronic flux in chemical reactions. Sci China Chem 54:1982–1988
Duarte F, Toro-Labbé A (2011) The mechanism of H2 activation by (amino)carbenes. J Phys Chem A 115:3050–3059
Hernández Mancera JP, Núñez-Zarur F, Gutiérrez-Oliva S, Toro-Labbé A, Vivas-Reyes R (2020) Diels-alder reaction mechanisms of substituted chiral anthracene: a theoretical study based on the reaction force and reaction electronic flux. J Comput Chem 41:2022–2032
Hernández-Mancera JP, Núñez-Zarur F, Vivas-Reyes R (2020) Diels-alder reactivity of a chiral anthracene template with symmetrical and unsymmetrical dienophiles: a DFT study. ChemistryOpen 9:748–761
Toro-Labbé A (1999) Characterization of chemical reactions from the profiles of energy, chemical potential, and hardness. J Phys Chem A 103:4398–4403
Politzer P, Toro-Labbé A, Gutiérrez-Oliva S, Herrera B, Jaque P, Concha MC, Murray JS (2005) The reaction force: three key points along an intrinsic reaction coordinate. J Chem Sci 117:467–472
Gutiérrez-Oliva S, Herrera B, Toro-Labbé A, Chermette H (2005) On the mechanism of hydrogen transfer in the HSCH(O) ⇄ (S)CHOH and HSNO ⇄ SNOH reactions. J Phys Chem A 109:1748–1751
Politzer P, Murray JS, Yepes D, Jaque P (2014) Driving and retarding forces in a chemical reaction. J Mol Model 20:2351
Yepes D, Donoso-Tauda O, Pérez P, Murray JS, Politzer P, Jaque P (2013) The reaction force constant as an indicator of synchronicity/ nonsynchronicity in [4+2] cycloaddition processes. Phys Chem Chem Phys 15:7311–7320
Burda JV, Toro-Labbé A, Gutiérrez-Oliva S, Murray JS, Politzer P (2007) Reaction force decomposition of activation barriers to elucidate solvent effects. J Phys Chem A 111:2455–2457
Toro-Labbé A, Gutiérrez-Oliva S, Murray JS, Politzer P (2009) The reaction force and the transition region of a reaction. J Mol Model 15:707–7110
Gutiérrez-Oliva S, Herrera B, Toro-Labbé A (2018) An extension of the marcus equation: the marcus potential energy function. J Mol Model 24:1–6
Gutiérrez‐Oliva S, Díaz S, Toro‐Labbé A (2021) Chemical Reactivity in Confined Systems: Theory, Modelling and Applications, 81–97
Cárdenas-Jirón GI, Toro-Labbé A, Bock CW, Maruani J (1995) Characterization of rotational isomerization processes in monorotor molecules. Struct Dyn Non-Rigid Mol Syst 12:97–120
Martínez J, Toro-Labbé A (2004) Energy and chemical force profiles from the Marcus equation. Chem Phys Lett 392:132–139
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338
Marcus RA (1964) Chemical and electrochemical electron-transfer theory. Annu Rev Phys Chem 15:155–196
Marcus RA (1993) Electron transfer reactions in chemistry, theory and experiment. Rev Mod Phys 65:599
Van Zeist WJ, Koers AH, Wolters LP, Bickelhaupt FM (2008) Reaction coordinates and the transition-vector approximation to the IRC. J Chem Theory Comput 4:920–928
Van Zeist WJ, Bickelhaupt FM (2010) The activation strain model of chemical reactivity. Org Biomol Chem 8:3118–3127
Wolters LP, Bickelhaupt FM (2015) The activation strain model and molecular orbital theory. Wiley Interdiscip Rev Comput Mol Sci 5:324–343
Kubelka J, Bickelhaupt FM (2017) Activation strain analysis of SN2 reactions at C, N, O, and F centers. J Phys Chem A 121:885–891
Díaz S, Brela MZ, Gutiérrez-Oliva S, Toro-Labbé A, Michalak A (2017) ETS-NOCV decomposition of the reaction force: the HCN/CNH isomerization reaction assisted by water. J Comput Chem 38:2076–2087
Barrales-Martínez C, Cortés-Arriagada D, Gutiérrez-Oliva S (2018) Molecular hydrogen formation in the interstellar medium: the role of polycyclic aromatic hydrocarbons analysed by the reaction force and activation strain model. Mon Not R Astron Soc 481:3052–3062
Durán R, Herrera B (2019) Theoretical study of the mechanism of catalytic enanteoselective N-H and O-H insertion reactions. J Phys Chem A 124:2–11
Herrera B, Toro-Labbé A (2007) The role of reaction force and chemical potential in characterizing the mechanism of double proton transfer in the adenine-uracil complex. J Phys Chem A 111:5921–5926
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Pearson RG (1985) Absolute electronegativity and absolute hardness of Lewis acids and bases. J Am Chem Soc 107:6801–6806
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113
Janak JF (1978) Proof that dE/dn_i = e_i in density-functional theory. Phys Rev B 18:7165
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874
Gutiérrez-Oliva S, Forero-Girón AC, Villegas-Escobar N, Toro-Labbé A (2022) On the mechanisms of chemical reactions. Concept Density Funct Theory Towar New Chem React Theory 2:463–479
Brauer B, Kesharwani MK, Martin JML (2014) Some observations on counterpoise corrections for explicitly correlated calculations on noncovalent interactions. J Chem Theory Comput 10:3791–3799
Pearson RG (2005) Chemical hardness and density functional theory. J Chem Sci 117:369–377
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Jr JAM, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016)
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for ain group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements : two new functionals and systematic testing of four m06-class functionals and 12 other fun. Theor Chem Acc 120:215–241
Krishnan RBJS, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Jensen F (2007) Introduction to computational chemistry, 2a edn. The Atrium, Southern Gate
Fukui K, Kato S, Fujimoto H (1975) Constituent analysis of the potential gradient along a reaction coordinate. Method and an application to CH 4 + T reaction. J Am Chem Soc 97:1–7
Svatunek D, Houk KN (2019) AutoDIAS: A python tool for an automated distortion/interaction activation strain analysis. J Comput Chem 40:2509–2515
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Acknowledgements
All authors gratefully acknowledge the financial support from ANID (Chile) through Project FONDECYT N0. 1201617. JHM and RVR acknowledge financial support provided by Ministry of Science, Technology and Innovation (Colombia) for a Postdoctoral grant at the Universidad de Cartagena (Cartagena, Colombia). This paper is dedicated to our dear friend, Professor Pratim K. Chattaraj for his 65th anniversary. Happy Birthday Pratim!!
Funding
ANID (Chile) through Project FONDECYT No. 1201617.
Author information
Authors and Affiliations
Contributions
Jennifer Hernandez-Mancera made most calculations and prepared the figures and wrote the manuscript; Ricardo Vivas-Reyes suggested the reactions that are studied in this paper, he helped preparing the figures and discussing the results; Soledad Gutiérrez-Oliva was involved in the benchmark of the methodologies, she helped with the analysis of results and reviewed the manuscript and discussed the results; Bárbara Herrera helped with the calculations suggesting methodologies, she was involved in the analysis of results, reviewed the manuscript; Alejandro Toro-Labbé proposed the use of theoretical and conceptual tools for analysis; was in charge of the structure and organization of the paper and performing exhaustive reviews of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández-Mancera, J.P., Vivas-Reyes, R., Gutiérrez-Oliva, S. et al. Digging on the mechanism of some Diels–Alder reactions: the role of the reaction electronic flux. Theor Chem Acc 142, 64 (2023). https://doi.org/10.1007/s00214-023-03019-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03019-3