Abstract
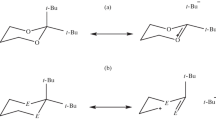
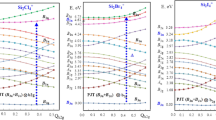
Claisen and Cope rearrangements are [3,3]-sigmatropic rearrangements thermally activated that occur through six-membered transition states. Although extensively investigated for decades, little is known about the magnetochemistry of these rearrangements. In view of this, we carried out an investigation based on chemical-computational models through methods based on Density Functional Theory, QTAIM, Multicenter Bond Order, NCI, GIAO, and GIMIC. We demonstrated that CCR mechanisms are concerted in which the 6-membered cyclic transition states present high aromaticity character. The molar and anisotropic susceptibility, NICS(1), and NICSzz signals were verified to have a good correlation with J (nA T−1). However, these indices demonstrated insufficient to evaluate the magnitudes of aromaticity in these transition states. Among the magnetic and topological descriptors applied in this work, the magnetically induced current density (J) proved, this to be an excellent strategy for theoretically estimative of the aromaticity of the transition states involved in the investigated rearrangements. Among the rearrangements with chair conformation, it was noted that the higher aromaticity is associated with the less favoured kinetics. For chair conformations, Claisen rearrangement (TS2) presents J = 8.63 nA T−1 and Cope (TS4) presents J = 10.43 nA T−1. However, the following order of aromaticity TS4 > TS2 > TS3 > TS1, with high paramagnetic currents in the boat conformations reducing the total current in the ring, suggests that it is not possible to establish a direct correlation between aromaticity and the kinetics of the Cope and Claisen rearrangements, since the most stable transition estate geometries are not necessarily the most aromatic.







Similar content being viewed by others
References
Woodward RB, Hoffmann R (1969) The conservation of orbital symmetry. Angew Chem Int Ed Engl 8:781–853. https://doi.org/10.1002/anie.196907811
Cope AC, Hardy EM (1940) The introduction of substituted vinyl groups. V. A rearrangement involving the migration of an allyl group in a three-carbon system1. J Am Chem Soc 62:441–444. https://doi.org/10.1021/ja01859a055
Claisen L (1912) Über Umlagerung von Phenol-allyläthern in C-Allyl-phenole. Ber Dtsch Chem Ges 45:3157–3166. https://doi.org/10.1002/cber.19120450348
Ilardi EA, Stivala CE, Zakarian A (2009) [3,3]-Sigmatropic rearrangements: recent applications in the total synthesis of natural products. Chem Soc Rev 38:3133–3148. https://doi.org/10.1039/B901177N
Nowicki J (2000) Claisen, Cope and related rearrangements in the synthesis of flavour and fragrance compounds. Molecules 5:1033–1050
Freitas JJR, Avelino RA, Mata MMS et al (2017) Rearranjos de claisen mais usados em síntese orgânica: uma revisão. Revista Virtual de Quimica 9
Williams RV (2001) Homoaromaticity. Chem Rev 101:1185–1204. https://doi.org/10.1021/cr9903149
Graulich N (2011) The Cope rearrangement-the first born of a great family. Wiley Interdiscip Rev Comput Mol Sci. https://doi.org/10.1002/wcms.17
Zelentsov S, Hessel V, Shahbazali E, Noël T (2014) The Claisen rearrangement–part 1: mechanisms and transition states, revisited with quantum mechanical calculations and ultrashort pulse spectroscopy. ChemBioEng Rev 1:23–240
Rzepa HS (2007) The aromaticity of pericyclic reaction transition states. J Chem Educ. https://doi.org/10.1021/ed084p1535
Jiao H, von Schleyer PR (1998) Aromaticity of pericyclic reaction transition structures: magnetic evidence. J Phys Org Chem 11:655–662
Morao I, Cossío FP (1999) A simple ring current model for describing in-plane aromaticity in pericyclic reactions. J Org Chem. https://doi.org/10.1021/jo981862+
Jiao H, von RaguéSchleyer P (1995) The Cope rearrangement transition structure is not diradicaloid, but is it aromatic? Angew Chem Int Ed English. https://doi.org/10.1002/anie.199503341
Domingo LR, Ríos-Gutiérrez M, Chamorro E, Pérez P (2016) Aromaticity in pericyclic transition state structures? A critical rationalisation based on the topological analysis of electron density. ChemistrySelect 1:6026–6039. https://doi.org/10.1002/slct.201601384
Solà M (2017) Why aromaticity is a suspicious concept? Why? Front Chem. https://doi.org/10.3389/FCHEM.2017.00022/FULL
Solà M, Feixas F, Jiménez-Halla JOC et al (2010) A critical assessment of the performance of magnetic and electronic indices of aromaticity. Symmetry 2:1156–1179
Chen Z, Wannere CS, Corminboeuf C et al (2005) Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem Rev 105:3842–3888
Schleyer PV, Maerker C, Dransfeld A et al (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318. https://doi.org/10.1021/ja960582d
Gershoni-Poranne R, Stanger A (2014) The NICS-XY-scan: identification of local and global ring currents in multi-ring systems. Chem Eur J 20:100. https://doi.org/10.1002/chem.201304307
Gershoni-Poranne R, Stanger A (2015) Magnetic criteria of aromaticity. Chem Soc Rev 44:6597–6615. https://doi.org/10.1039/C5CS00114E
Gershoni-Poranne R, Stanger A (2021) 4—NICS-Nucleus-independent Chemical Shift. In: Fernandez I (ed) Aromaticity. Elsevier, pp 99–154
Schleyer PVR, Wu JI, Cossío FP, Fernández I (2014) Aromaticity in transition structures. Chem Soc Rev 43:4909–4921
Fliegl H, Jusélius J, Sundholm D (2016) Gauge-origin independent calculations of the anisotropy of the magnetically induced current densities. J Phys Chem A 120:5658–5664. https://doi.org/10.1021/acs.jpca.6b03950
Taubert S, Sundholm D, Jusélius J (2011) Calculation of spin-current densities using gauge-including atomic orbitals. J Chem Phys 134:054123-054123–054212. https://doi.org/10.1063/1.3549567
Patra SG, Mandal N (2020) Aromaticity of N-heterocyclic carbene and its analogues: magnetically induced ring current perspective. Int J Quantum Chem. https://doi.org/10.1002/qua.26152
Fliegl H, Sundholm D, Taubert S et al (2009) Magnetically induced current densities in aromatic, antiaromatic, homoaromatic, and nonaromatic hydrocarbons. J Phys Chem A 113:8668–8676. https://doi.org/10.1021/jp9029776
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2827. https://doi.org/10.1063/1.477924
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542. https://doi.org/10.1063/1.481224
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37:785–789. https://doi.org/10.1103/physrevb.37.785
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Woon DE, Dunning TH (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371. https://doi.org/10.1063/1.464303
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260. https://doi.org/10.1021/ja00179a005
Sundholm D, Fliegl H, Berger RJF (2016) Calculations of magnetically induced current densities: theory and applications. Wiley Interdiscip Rev Comput Mol Sci 6:639–678. https://doi.org/10.1002/wcms.1270
Ahrens J, Geveci B, Law C (2005) Paraview: an end-user tool for large data visualization. Vis Handb. https://doi.org/10.1016/B978-012387582-2/50038-1
Pastorczak E, Corminboeuf C (2017) Perspective: found in translation: Quantum chemical tools for grasping non-covalent interactions. J Chem Phys 146:120901. https://doi.org/10.1063/1.4978951
Contreras-García J, Boto RA, Izquierdo-Ruiz F et al (2016) A benchmark for the non-covalent interaction (NCI) index or… is it really all in the geometry? Theor Chem Acc 135:242. https://doi.org/10.1007/s00214-016-1977-7
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev. https://doi.org/10.1021/cr00005a013
Bader RFW (1994) Atoms in molecules: a quantum theory, 22nd edn. Oxford University Press, Oxford
Keith T (2019) AIMAll, Version 19.10.12
Contreras-García J, Johnson ER, Keinan S et al (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput. https://doi.org/10.1021/ct100641a
Laplaza R, Peccati F, Arias-Olivares D, Contreras-García J (2021) 14 Visualizing non-covalent interactions with NCIPLOT. In: Grabowsky S (ed). De Gruyter, pp 353–378
Boto RA, Peccati F, Laplaza R et al (2020) NCIPLOT4: a new step towards a fast quantification of noncovalent interactions. ChemRxiv
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Frisch MJ, Trucks GW, Schlegel HB et al. Gaussian∼09 Revision D.01
Adrienko GA (2015) ChemCraft, V. 1.8
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218. https://doi.org/10.1002/jcc.540030212
Dominikowska MJP (2012) EL: the new aromaticity measure based on one-electron density function. Struct Chem 23:1173–1183. https://doi.org/10.1007/s11224-011-9941-6
Wiest O, Montiel DC, Houk KN (1997) Quantum mechanical methods and the interpretation and prediction of pericyclic reaction mechanisms. J Phys Chem A 101:8378–8388
Dewar MJS (1966) A molecular orbital theory of organic chemistry-VIII: romaticity and electrocyclic reactions. Tetrahedron. https://doi.org/10.1016/S0040-4020(01)82171-2
Boto RA, Peccati F, Laplaza R et al (2020) NCIPLOT4: fast, robust, and quantitative analysis of noncovalent interactions. J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.0c00063
Mandado M, González-Moa MJ, Mosquera RA (2007) Characterization of pericyclic reactions using multicenter electron delocalization analysis. ChemPhysChem 8:696–702. https://doi.org/10.1002/cphc.200600682
Noorizadeh S, Shakerzadeh E (2010) Shannon entropy as a new measure of aromaticity, Shannon aromaticity. Phys Chem Chem Phys 12:4742–4749. https://doi.org/10.1039/B916509F
Acknowledgements
T.S.C. and G.F.M. acknowledges CAPES/PPGQ-UnB for PhD scholarship. S.F. de A.M. acknowledges CNPq for the scholarship (Grant 165726/2020-2). D.A.C.F. is grateful to the SESu/MEC/PETQuímica-UnB for the tutor fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the full development of this article.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castro, T.S., Martins, G.F., de Alcântara Morais, S. et al. Aromaticity of Cope and Claisen rearrangements. Theor Chem Acc 142, 40 (2023). https://doi.org/10.1007/s00214-023-02975-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-02975-0