Abstract
The 1H, 13C, 15N and 29Si chemical shifts of three trimethylsilyl-1H-pyrazoles were calculated and compared with literature results; the calculations were carried out at the GIAO/B3LYP/6–311 + + G(d,p) level resulting in a very good agreement that allows to predict with confidence the missing experimental values. The prototropic barrier of 4-trimethylsilyl-1H-pyrazole (1) as well as the silylotropic barriers of 1-trimethylsilyl-1H-pyrazole (2) and 1-trimethylsilyl-4-methyl-1H-pyrazole (3) were also calculated and the mechanism was established, the accordance with the experimental values being satisfactory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dynamic phenomena are one of the essential aspects of chemistry; some of these phenomena occur without breaking/creating bonds, such as the conformational analysis of molecules [1], while others involve the building and breaking of bonds, the most known being prototropic tautomerism [2]. These processes are studied by dynamic NMR the technique being called DNMR.
Although prototropic tautomerism is important in general organic chemistry, for instance in β-diketones [3], most results come from heterocycles [2], particularly from azoles that have a N-substituted nitrogen atom and a N-unsubstituted one. In pyrazoles (but also in triazoles and tetrazoles), these nitrogen atoms occupy contiguous positions that facilitate the transfer of the migrating atom.
Proton transfer is by far the most common process, but nonetheless other groups can also migrate, among them, silyl groups like the trimethylsilyl (TMS) [4]. In 1998, Larina et al. reported a large series of NMR data in a paper where they wrote, "C- and N-trimethylsilylazole derivatives were studied by 1H, 13C and 29Si NMR spectroscopy. Degenerated prototropic tautomerism of 4-trimethylsilyl-pyrazole (1) in methanol and the silylotropy of 1-trimethylsilyl-4-methylpyrazole (3) in a neat liquid were investigated for the first time" [5]. [Compound 2 was also studied in this paper (Scheme 1).]
2 Computational details
Density functional theory (DFT) calculations were carried out using the Becke, three-parameter, Lee, Yang and Parr (B3LYP) functional [6,7,8] together with the 6–311 + + G(d,p) basis set [9, 10]. Frequency calculations were carried out to verify that the structures obtained correspond to energetic minima (I = 0) or to transition states (TS, I = 1): see Supplementary Information for the geometries of the minima and the TS.
Absolute shieldings were calculated within the GIAO approximation [11]. Empirical equations were used to transform the 1H, 13C, 15N and 29Si absolute shieldings into chemical shifts [12,13,14]. All these calculations have been carried out with the Gaussian 16 program [15].
3 Results and discussion
There are three compounds under study:
-
4-Trimethylsilyl-1H-pyrazole (1). This compound was first prepared by Birkofer [16] and studied thoroughly by him [17,18,19,20] and other authors [21,22,23,24,25,26]. (The X-ray structure of 1 has been determined [25].)
-
1-Trimethylsilyl-1H-pyrazole (2). Again Birkofer prepared for the first time in 1960 [27], we reported the synthesis of 2 in 1968 [28]. The subsequent syntheses were due to O'Brien [29] and to Wrackmeyer [30, 31]. We have devoted several papers to the synthesis and study of this compound [32,33,34,35,36,37,38].
-
4-Methyl-1-trimethylsilyl-1H-pyrazole (3). Reported for the first and only time by Larina et al. [5].
3.1 Static part
We have reported in Table 1 the calculated and experimental NMR values concerning compounds 1, 2 and 3. A column called "Prototropy" reports the GIAO calculated values when averaged by prototropy and silylotropy; this affects H3/H5, C3/C5 and N1/N2, for instance, (7.47 + 7.17)/2 = 7.32 ppm. In the case of compound 1 in CD3OD at low temperature (–90 ºC), only H3, H5 and SiMe3 were reported to appear at 7.70, 7.56 and 0, 21 ppm, respectively.
The data of Table 1 were analyzed statistically using the calculated mean values; with regard to CDCl3, the other solvents (CCl4, CD3OD and "neat liquid") do not modify the values in a significant way; on the other hand, the NH proton of compound 1 differs significantly from the GIAO calculated value in the gas phase 9.06 vs. 12.97 and 14.58 ppm), a well-known fact [41]. In the following equation, NH variable corresponds to these differences but statistically calculated.
The resulting equation is Exp. (ppm) = (1.01 ± 0.04) GIAO (ppm) + (4.7 ± 1.6) NH (ppm), n = 50, R2 = 0.999. The solvent effect on the NH is 4.7 ppm. Since the slope is 1.01, the missing experimental values of Table 1 should be very close to the calculated ones.
3.2 Dynamic part: barriers
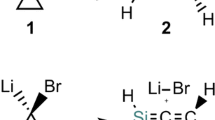
We have summarized in Table 2 the experimental barriers determined by Larina et al. by DNMR for the compounds of Scheme 1 [42]. The barrier of compound 2 (2a/2b equilibrium) was measured by O'Brien & Hrung [29] who reported a value of 133.9 kJ mol–1. Larina et al. [22, 40, 42] using their DNMR data calculate a barrier of 96.7 kJ mol–1, using the temperature of coalescence TC and the equation that relates the barrier energy to the temperature of coalescence: ΔG‡C = 19.12 * TC * (10.32 + log TC/kC), the temperature of coalescence TC is 438 K, kC = (π * Δν)/√2, Δν = 11.6 Hz (kC = 25.8 s–1), and consequently, ΔG‡C = 96.7 kJ·mol–1.
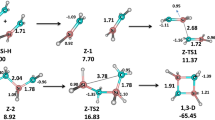
It is well known that the direct proton transfer in NH-pyrazoles is forbidden resulting in very high barriers; solvent molecules or other NH-pyrazole molecules are necessary to facilitate the transfer [45,46,47,48,49]; these auxiliaries must have centers able to establish hydrogen bonds, either HB acceptors, HB donors or both, like water. To test the reliability of our approach, we have calculated those of the parent pyrazole, as shown in Fig. 1 (all values in kJ mol–1). Similar structures for the unsubstituted pyrazole were published by Oziminski [49] who reported at the MP2/ B3LYP/6-311 + + G(d,p) level a barrier of ΔE = 81.6 kJ mol–1 to compare with ΔE = 82.2 kJ mol–1 of Fig. 1, right side. Note that an experimental study has demonstrated the role of water in the prototropy of phenyl-methyl-pyrazole [50].
Then, we have carried out the same calculations on 1 obtaining very similar results, i.e., according to the calculations in the gas phase the effect of the 4-trimethylsilyl is insignificant, as shown in Fig. 2. Experimentally, there is a noticeable decrease, from 61.9 to 49.8 kJ mol–1 (12.1 kJ mol–1).
Continuum solvation effects, estimated with the PCM approximation [51], reduce the barrier slightly (Table 3), but it remains overestimated. Considering that the presence of two water molecules continues to be a model, the results are satisfactory. We have also calculated the value with methanol instead of water and PCM/methanol (Fig. 3 and Table 3), obtaining a lower value.
The characteristic out-of-plane TSs of the silylotropy [4] (Fig. 4) are very similar for 2 and 3. The calculated barriers show that the 4-methyl group produces almost no effect, and that of 3 is a slightly lower in agreement with the experimental values (Table 2), but overestimated, ratio calculated/experimental, 1.07 for 2 and 1.09 for 3.
4 Conclusions
Whereas the agreement between calculated and experimental chemical shifts for the three derivatives, 4-trimethylsilyl-1H-pyrazole (1), 1-trimethylsilyl-1H-pyrazole (2) and 1-trimethylsilyl-4-methyl-1H-pyrazole (3), was expected due to our previous experience on these relationships, the part concerning the barriers to the dynamic processes (prototropy and silylotropy) is more complex. When the migrating group is the trimethylsilyl, the calculations do not involve any particular problem and the results are good. On the other hand, prototropy needs the assistance of solvent molecules that other authors [49, 52] and ourselves [45,46,47,48] have modeled with water molecules, which is only an acceptable simplification.
References
Eliel EL, Willen SH, Mander LN (1994) Stereochemistry of organic compounds. John Wiley & Sons, New York
Elguero J, Marzin C, Katritzky AR, Linda P (1976) The tautomerism of heterocycles. Academic Press, New York
Claramunt RM, López C, Lott S, Santa María MD, Alkorta I, Elguero J (2005) Helv Chim Acta 88:1931–1942
Alkorta I, Elguero J (2005) Heteroatom Chem 16:628–636
Larina LI, Sorokin MS, Albanov AI, Elokhina VN, Protsuk NI, Lopyrev VA (1998) Magn Reson Chem 36:110–115
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1988) Phys Rev A 38:3098–3100
Becke AD (1993) J Chem Phys 98:5648–5652
Ditchfield R, Hehre WJ, Pople JA (1971) J Chem Phys 54:724–728
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269
London F (1937) J Phys Radium 8:397–409
Silva AMS, Sousa RMS, Jimeno ML, Blanco F, Alkorta I, Elguero J (2008) Magn Reson Chem 46:859–864
Blanco F, Alkorta I, Elguero J (2007) Magn Reson Chem 45:797–800
Elguero J, Alkorta I, Claramunt RM, Cabildo P, Cornago P, Farrán MA, García MA, López C, Pérez-Torralba M, Santa María D, Sanz D (2013) Chem Heterocycl Comp 49:177–202
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT
Birkofer L, Franz M (1967) Chem Ber 100:2681–2684
Birkofer L, Franz M (1971) Chem Ber 104:3062–3068
Birkofer L, Franz M (1972) Chem Ber 105:1759–1767
Birkofer L, Stuhl O (1980) Top Curr Chem 88:33–88
Birkofer L, Kühn T (1981) Chem Ber 114:2293–2299
Effenberger F, Krebs A (1984) J Org Chem 49:4687–4695
Lopyrev VA, Larina LI, Voronkov G (2001) Russ J Org Chem 37:149–193
Gowenlock BG, Richter-Addo GB (2004) Chem Rev 104:3315–3340
Seven Ö, Popp S, Bolte M, Lerner HW, Wagner M (2014) Dalton Trans 43:8241–8253
Pejic M, Popp S, Bolte M, Wagner M, Lerner HW (2014) Z Naturforsch 69b:83–97
Onodera S, Kochi T, Kakiuchi F (2019) J Org Chem 84:6508–6515
Birkofer L, Richter P, Ritter A (1960) Chem Ber 93:2804–2809
Elguero J, Rivière-Baudet M, Satgé J (1968) C R Acad Sci Paris 266:44–47
O’Brien DH, Hrung CP (1971) J Organomet Chem 27:185–193
Nöth H, Wrackmeyer B (1974) Chem Ber 107:3070–3088
Wrackmeyer B (1984) Spectrochim Acta 40A:963–977
Gasparini JP, Gassend R, Maire JC, Elguero J (1980) J Organomet Chem 188:141–150
Gasparini JP, Gassend R, Maire JC, Elguero J (1981) J Organomet Chem 208:309–315
Begtrup M, Elguero J, Faure R, Camps P, Estopá C, Ilavsky D, Fruchier A, Marzin C, Mendoza JD (1988) Magn Reson Chem 26:134–151
Elguero J, Claramunt RM, López C, Sanz D (1993) Afinidad 448:383–388
Claramunt RM, Sanz D, Santa María MD, Jiménez JA, Jimeno ML, Elguero J (1998) Heterocycles 47:301–314
Begtrup M, Balle T, Claramunt RM, Sanz D, Jiménez JA, Mó O, Yáñez M, Elguero J (1998) J Mol Struct (Theochem) 453:255–273
Claramunt RM, Sanz D, Alkorta I, Elguero J (2005) Magn Reson Chem 43:985–991
Claramunt RM, Sanz D, López C, Jiménez JA, Jimeno ML, Elguero J, Fruchier A (1997) Magn Reson Chem 35:35–75
Lopyrev VA, Larina LI, Albanov AI, Sorokin MS, Dolgushin GV (1996) Russ Chem Bull 45:2861–2862
Alkorta I, Claramunt RM, Elguero J, Gutiérrez-Puebla E, Monge MA, Reviriego F, Roussel C (2022) J Org Chem 87:5866–5881
Larina LI (2021) Adv Heterocycl Chem 133:1–63
Litchman WM (1979) J Am Chem Soc 101:545–547
Minkin VI, Garnovskii AD, Elguero J, Katritzky AR, Denisko OV (2000) Adv Heterocycl Chem 76:157–323
Catalán J, de Paz JLG, Sánchez-Cabezudo M, Elguero J (1986) Bull Soc Chim Fr 429–435
Alkorta I, Elguero J (1998) J Chem Soc Perkin Trans 2:2497–2503
Alkorta I, Rozas I, Elguero J (1998) J Chem Soc Perkin Trans 2:2671–2675
Trujillo C, Sánchez-Sanz G, Alkorta I, Elguero J (2015) ChemPhysChem 16:2140–2150
Oziminski WP (2016) Struct Chem 27:1845–1854
Bensaude O, Chevrier M, Dubois JE (1978) Tetrahedron 34:2259–2262
Tomasi J, Persico M (1994) Chem Rev 94:2027–2094
Secrieru A, O’Neil PM, Cristiano MLS (2020) Molecules 25:42
Acknowledgements
This work was carried out with financial support from the Ministerio de Ciencia, Innovación y Universidades (PGC2018-094644-B-C22 and PID2021-125207NB-C32), Comunidad de Madrid (P2018/EMT-4329). The authors thank the CTI (CSIC) for their continued computational support.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
The three authors wrote the main manuscript text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Claramunt, R.M., Elguero, J. & Alkorta, I. A theoretical study of dynamic processes observed in trimethylsilyl-1H-pyrazoles: prototropy and silylotropy. Theor Chem Acc 141, 78 (2022). https://doi.org/10.1007/s00214-022-02936-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02936-z