Abstract
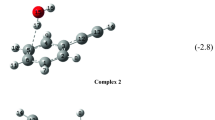
The formation of a peptide bond from carboxylic acid and amine from the reactants taken separately in the gas phase with no catalytic surface constitutes an interesting process from an exobiological point of view. We investigated the reaction between acetic acid (CH3–COOH) and methylamine (CH3–NH2), alone and with the presence of one to five water molecules, at the LC-ωPBE/6-311++G(d,p) level of theory, with the GD3BJ Empirical Dispersion. Starting from the idea that the reaction begins with the formation of a non-covalent complex between the reagents, we first identified the main structures of non-covalent complexes that can be formed between both reagents without hydration, by maximizing interactions between complementary sites of both partners. This study led to the identification of a (CH3–COOH):(CH3–NH2) complex, with a stabilization energy of 14.22 kJ/mol, that may correspond to a preliminary step towards the formation of a peptide-like bond.

















Similar content being viewed by others
References
Perez JE, Kumar M, Francisco JS, Sinha A (2017) Oxygenate-induced tuning of aldehyde-amine reactivity and its atmospheric implications. J Phys Chem A 121(5):1022–1031
Dong ZG, Xu F, Long B (2018) The energetics and kinetics of the CH3CHO+(CH3) 2NH/CH3NH2 reactions catalyzed by a single water molecule in the atmosphere. Comput Theor Chem 1140:7–13
Louie MK, Francisco JS, Verdicchio M, Klippenstein SJ, Sinha A (2016) Dimethylamine addition to formaldehyde catalyzed by a single water molecule: a facile route for atmospheric carbinolamine formation and potential promoter of aerosol growth. J Phys Chem A 120(9):1358–1368
Riffet V, Frison G, Bouchoux G (2018) Quantum-chemical modeling of the first steps of the Strecker synthesis: from the gas-phase to water solvation. J Phys Chem A 122(6):1643–1657
Tan XF, Zhang L, Long B (2020) New mechanistic pathways for the formation of organosulfates catalyzed by ammonia and carbinolamine formation catalyzed by sulfuric acid in the atmosphere. Phys Chem Chem Phys 22(16):8800–8807
Liu JY, Long ZW, Mitchell E, Long B (2021) New mechanistic pathways for the reactions of formaldehyde with formic acid catalyzed by sulfuric acid and formaldehyde with sulfuric acid catalyzed by formic acid: formation of potential secondary organic aerosol precursors. ACS Earth Space Chem 5(6):1363–1372
Galloway MM, Powelson MH, Sedehi N, Wood SE, Millage KD, Kononenko JA, Rynaski AD, De Haan DO (2014) Secondary organic aerosol formation during evaporation of droplets containing atmospheric aldehydes, amines, and ammonium sulfate. Environ Sci Technol 48(24):14417–14425
Dong ZG, Xu F, Mitchell E, Long B (2021) Trifluoroacetaldehyde aminolysis catalyzed by a single water molecule: an important sink pathway for trifluoroacetaldehyde and a potential pathway for secondary organic aerosol growth. Atmos Environ 249:118242
Colzi L, Rivilla VM, Beltrán MT, Jiménez-Serra I, Mininni C, Melosso M, Cesaroni R, Fontani F, Lorenzani A, Sanchez-Monge A et al (2021) The GUAPOS project II. A comprehensive study of peptide-like bond molecules. arXiv preprint arXiv:2107.11258
Georgelin T, Jaber M, Bazzi H, Lambert JF (2013) Formation of activated biomolecules by condensation on mineral surfaces–a comparison of peptide bond formation and phosphate condensation. Origins Life Evol Biospheres 43(4):429–443
Rimola A, Tosoni S, Sodupe M, Ugliengo P (2006) Does silica surface catalyse peptide bond formation? New Insights from First-Principles Calculations. ChemPhysChem 7(1):157–163
Rimola A, Sodupe M, Ugliengo P (2019) Role of mineral surfaces in prebiotic chemical evolution. In silico quantum mechanical studies. Life 9(1):10
Georgelin T, Akouche M, Jaber M, Sakhno Y, Matheron L, Fournier F, Méthivier C, Martra G, Lambert JF (2017) Iron (III) oxide nanoparticles as catalysts for the formation of linear glycine peptides. Eur J Inorg Chem 2017(1):198–211
Tielens F, Gierada M, Handzlik J, Calatayud M (2020) Characterization of amorphous silica based catalysts using DFT computational methods. Catal Today 354:3–18
Pantaleone S, Ugliengo P, Sodupe M, Rimola A (2018) When the surface matters: prebiotic peptide-bond formation on the TiO2 (101) anatase surface through periodic DFT-D2 simulations. Chem Eur J 24:16292–16301
Rufino VC, Pliego JR Jr (2020) The role of carboxylic acid impurity in the mechanism of the formation of aldimines in aprotic solvents. Comput Theor Chem 1191:113053
Parr RG, Yang W (1995) Density-functional theory of the electronic structure of molecules. Annu Rev Phys Chem 46(1):701–728
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106(14):4049–4050
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20:129–154
Politzer P, Murray JS (2020) Electrostatics and polarization in σ-and π-hole noncovalent interactions: an overview. ChemPhysChem 21(7):579–588
Politzer P, Murray JS, Clark T (2019) Explicit inclusion of polarizing electric fields in σ-and π-hole interactions. J Phys Chem A 123(46):10123–10130
Frontera A, Bauzá A (2017) Concurrent aerogen bonding and lone pair/anion–π interactions in the stability of organoxenon derivatives: a combined CSD and ab initio study. Phys Chem Chem Phys 19(44):30063–30068
Bijina PV, Suresh CH (2020) Molecular electrostatic potential reorganization theory to describe positive cooperativity in noncovalent trimer complexes. J Phys Chem A 124(11):2231–2241
Anjali BA, Suresh CH (2020) Absorption and emission properties of 5-phenyl tris (8-hydroxyquinolinato) M (III) complexes (M= Al, Ga, In) and correlations with molecular electrostatic potential. J Comput Chem 41(16):1497–1508
Sosorev A, Dominskiy D, Chernyshov I, Efremov R (2020) Tuning of molecular electrostatic potential enables efficient charge transport in crystalline azaacenes: a computational study. Int J Mol Sci 21(16):5654
Gadre SR, Suresh CH, Mohan N (2021) Electrostatic potential topology for probing molecular structure, bonding and reactivity. Molecules 26(11):3289
Pundlik SS, Gadre SR (1997) Structure and stability of DNA base trimers: an electrostatic approach. J Phys Chem B 101(46):9657–9662
Gadre SR, Bhadane PK (1997) Patterns in hydrogen bonding via electrostatic potential topography. J Chem Phys 107(14):5625–5626
Mohan N, Suresh CH, Kumar A, Gadre SR (2013) Molecular electrostatics for probing lone pair–π interactions. Phys Chem Chem Phys 15(42):18401–18409
Gadre SR, Kumar A (2015) Understanding lone pair-π interactions from electrostatic viewpoint. In: Noncovalent forces. Springer, Cham, pp 391–418
Kulkarni AD, Babu K, Gadre SR, Bartolotti LJ (2004) Exploring hydration patterns of aldehydes and amides: Ab initio investigations. J Phys Chem A 108(13):2492–2498
Ayers PW (2001) Strategies for computing chemical reactivity indices. Theoret Chem Acc 106(4):271–279
Geerlings P, Ayers PW, Toro-Labbé A, Chattaraj PK, De Proft F (2012) The Woodward–Hoffmann rules reinterpreted by conceptual density functional theory. Acc Chem Res 45(5):683–695
Geerlings P et al (2020) Conceptual density functional theory: status, prospects, issues. Theoret Chem Acc 139(2):1–18
Bacchus-Montabonel MC, Calvo F (2015) Nanohydration of uracil: emergence of three-dimensional structures and proton-induced charge transfer. Phys Chem Chem Phys 17:9629–9633
Pérez C, Neill JL, Muckle MT, Zaleski DP, Peña I, Lopez JC, Pate BH (2015) Water–water and water–solute interactions in microsolvated organic complexes. Angew Chem Int Ed 54(3):979–982
Blanco S, Pinacho P, López JC (2017) Structure and dynamics in formamide–(H2O) 3: A Water Pentamer Analogue. J Phys Chem Lett 8(24):6060–6066
Gruet S, Pérez C, Steber AL, Schnell M (2018) Where’s water? The many binding sites of hydantoin. Phys Chem Chem Phys 20(8):5545–5552
Derbali I, Thissen R, Alcaraz C, Romanzin C, Zins EL (2021) Study of the Reactivity of CH3COOH+ and COOH+ Ions With CH3NH2: evidence of the Formation of New Peptide-like C(O)-N Bonds. Manuscript No.: jp-2021-06630q Accepted for publication in J Phys Chem A
Sun F, Xie M, Zhang Y, Song W, Sun X, Hu Y (2021) Spectroscopic evidence of the C–N covalent bond formed between two interstellar molecules (ISM): acrylonitrile and ammonia. Phys Chem Chem Phys
Jeanvoine Y, Largo A, Hase WL, Spezia R (2018) Gas phase synthesis of protonated glycine by chemical dynamics simulations. J Phys Chem A 122(3):869–877
Scuderi D, Pérez-Mellor A, Lemaire J, Indrajith S, Bardaud JX, Largo A, Jeanvoine Y, Spezia R (2020) Infrared-Assisted Synthesis of Prebiotic Glycine. ChemPhysChem 21(6):503–509
Enrique-Romero J, Rimola A, Ceccarelli C, Ugliengo P, Balucani N, Skouteris D (2019) Reactivity of HCO with CH3 and NH2 on water ice surfaces. A comprehensive accurate quantum chemistry study. ACS Earth Space Chem 3(10):2158–2170
van der Rest G, Jensen LB, Azeim SA, Mourgues P, Audier HE (2004) Reactions of [NH3+·, H2O] with carbonyl compounds: a McLafferty rearrangement within a complex? J Am Soc Mass Spectrom 15(7):966–971
van der Rest G, Mourgues P, Nedev H, Audier HE (2002) A prototype for catalyzed amide bond cleavage: production of the [NH3, H2O]•+ dimer from ionized formamide and its carbene isomer. J Am Chem Soc 124(19):5561–5569
Politzer P, Murray JS (2018) The Hellmann–Feynman theorem: a perspective. J Mol Model 24(9):266
Bader RF, Carroll MT, Cheeseman JR, Chang C (1987) Properties of atoms in molecules: atomic volumes. J Am chem Soc 109(26):7968–7979
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21(3):1–10
Herbert JM (2021) Neat, simple, and wrong: debunking electrostatic fallacies regarding noncovalent interactions. J Phys Chem A
Gadre SR, Babu K, Rendell AP (2000) Electrostatics for exploring hydration patterns of molecules. 3. Uracil. J Phys Chem A 104(39):8976–8982
Sivanesan D, Babu K, Gadre SR, Subramanian V, Ramasami T (2000) Does a stacked DNA base pair hydrate better than a hydrogen-bonded one? An ab initio study. J Phys Chem A 104(46):10887–10894
Güssregen S, Matter H, Hessler G, Müller M, Schmidt F, Clark T (2012) 3D-QSAR based on quantum-chemical molecular fields: toward an improved description of halogen interactions. J Chem Inf Model 52(9):2441–2453
El Kerdawy A, Güssregen S, Matter H, Hennemann M, Clark T (2013) Quantum mechanics-based properties for 3D-QSAR. J Chem Inf Model 53(6):1486–1502
El Kerdawy A, Wick CR, Hennemann M, Clark T (2012) Predicting the sites and energies of noncovalent intermolecular interactions using local properties. J Chem Inf Model 52(4):1061–1071
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian, Inc., Wallingford CT
Vydrov OA, Scuseria GE (2006) Assessment of a long-range corrected hybrid functional. J Chem Phys 125(23):234109
Vydrov OA, Heyd J, Krukau AV, Scuseria GE (2006) Importance of short-range versus long-range Hartree–Fock exchange for the performance of hybrid density functionals. J Chem Phys 125(7):074106
Vydrov OA, Scuseria GE, Perdew JP (2007) Tests of functionals for systems with fractional electron number. J Chem Phys 126(15):154109
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32(7):1456–1465
Krishnan RBJS, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72(1):650–654
Goerigk L, Kruse H, Grimme S (2011) Benchmarking density functional methods against the S66 and S66x8 datasets for non-covalent interactions. ChemPhysChem 12(17):3421–3433
DiLabio GA, Johnson ER, Otero-de-la-Roza A (2013) Performance of conventional and dispersion-corrected density-functional theory methods for hydrogen bonding interaction energies. Phys Chem Chem Phys 15(31):12821–12828
Bader RF, Streitwieser A, Neuhaus A, Laidig KE, Speers P (1996) Electron delocalization and the Fermi hole. J Am Chem Soc 118(21):4959–4965
Wick CR, Clark T (2018) On bond-critical points in QTAIM and weak interactions. J Mol Model 24(6):1–9
Jabłoński M (2020) Counterintuitive bond paths: an intriguing case of the C (NO2) 3-ion. Chem Phys Lett 759:137946
Jabłoński M (2019) On the uselessness of bond paths linking distant atoms and on the violation of the concept of privileged exchange channels. ChemistryOpen 8(4):497
Suvitha A, Venkataramanan NS, Sahara R, Kawazoe Y (2019) A theoretical exploration of the intermolecular interactions between resveratrol and water: a DFT and AIM analysis. J Mol Model 25(3):1–11
Venkataramanan NS, Suvitha A, Kawazoe Y (2017) Intermolecular interaction in nucleobases and dimethyl sulfoxide/water molecules: A DFT, NBO, AIM and NCI analysis. J Mol Graph Model 78:48–60
Sathiyamoorthy VN (2021) Electronic structure, stability, and cooperativity of chalcogen bonding in sulfur dioxide and hydrated sulfur dioxide clusters: a DFT study and wave functional analysis
Grabowski SJ, Leszczynski J (2009) The enhancement of X-H⋯ π hydrogen bond by cooperativity effects–Ab initio and QTAIM calculations. Chem Phys 355(2–3):169–176
Derbali I, Zins EL, Alikhani ME (2019) What is the hydrophobic interaction contribution to the stabilization of micro-hydrated complexes of trimethylamine oxide (TMAO)? A joint DFT-D, QTAIM, and MESP study. J Mol Model 25(12):1–14
Zins EL (2020) Microhydration of a carbonyl group: how does the molecular electrostatic potential (MESP) impact the formation of (H2O) n:(R2C═ O) complexes? J Phys Chem A 124(9):1720–1734
Gao J, Seifert NA, Jäger W (2019) A microwave spectroscopic and ab initio study of keto–enol tautomerism and isomerism in the cyclohexanone–water complex. Phys Chem Chem Phys 21(24):12872–12880
Weinhold F, Klein RA (2012) What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions. Mol Phys 110(9–10):565–579
Akman F, Issaoui N, Kazachenko AS (2020) Intermolecular hydrogen bond interactions in the thiourea/water complexes (Thio-(H 2 O) n)(n= 1,…, 5): X-ray, DFT, NBO, AIM, and RDG analyses. J Mol Model 26:1–16
Grabowski SJ (2020) Triel bond and coordination of triel centres–Comparison with hydrogen bond interaction. Coord Chem Rev 407:213171
Scheiner S, Michalczyk M, Wysokiński R, Zierkiewicz W (2020) Structures and energetics of clusters surrounding diatomic anions stabilized by hydrogen, halogen, and other noncovalent bonds. Chem Phys 530:110590
Gholami S, Aarabi M, Grabowski SJ (2021) Coexistence of intra-and intermolecular hydrogen bonds: salicylic acid and salicylamide and their thiol counterparts. J Phys Chem A 125(7):1526–1539
Hobza P, Müller-Dethlefs K (2010) Non-covalent interactions: theory and experiment (No. 2). Royal Society of Chemistry
Gadre SR, Yeole SD, Sahu N (2014) Chem Rev 114:12132–12173
Murray JS, Sen K (1996) Molecular electrostatic potentials: concepts and applications. Elsevier Science, Amsterdam
Kolar MH, Hobza P (2016) Computer modeling of halogen bonds and other σ-hole interactions. Chem Rev 116(9):5155–5187
Bauzá A, Mooibroek TJ, Frontera A (2015) The bright future of unconventional σ/π-hole interactions. ChemPhysChem 16(12):2496–2517
Remya K, Suresh CH (2015) Non-covalent intermolecular carbon–carbon interactions in polyynes. Phys Chem Chem Phys 17(40):27035–27044
Lv SS, Liu YR, Huang T, Feng YJ, Jiang S, Huang W (2015) Stability of hydrated methylamine: structural characteristics and H2N··· H–O hydrogen bonds. J Phys Chem A 119(16):3770–3779
Krishnakumar P, Maity DK (2017) Microhydration of neutral and charged acetic acid. J Phys Chem A 121(2):493–504
Kang NS, Kang YK (2017) Assessment of CCSD (T), MP2, and DFT methods for the calculations of structures and interaction energies of the peptide backbone with water molecules. Chem Phys Lett 687:23–30
Calvo F, Bacchus-Montabonel MC (2018) Size-induced segregation in the stepwise microhydration of hydantoin and its role in proton-induced charge transfer. J Phys Chem A 122(6):1634–1642
Calvo F, Bacchus-Montabonel MC, Clavaguéra C (2016) Stepwise hydration of 2-aminooxazole: theoretical insight into the structure, finite temperature behavior and proton-induced charge transfer. J Phys Chem A 120(15):2380–2389
Kalai C, Zins EL, Alikhani ME (2017) A theoretical investigation of water–solute interactions: from facial parallel to guest–host structures. Theoret Chem Acc 136(4):48
Derbali I, Thissen R, Alcaraz C, Romanzin C, Zins EL Study of the reactivity of CH3COOH+• and COOH+ ions with CH3NH2: Evidence of the formation of new peptide C(O)-N bonds. Submitted for publication in the Journal of Physical Chemistry A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Derbali, I., Aroule, O., Hoffmann, G. et al. On the relevance of the electron density analysis for the study of micro-hydration and its impact on the formation of a peptide-like bond. Theor Chem Acc 141, 34 (2022). https://doi.org/10.1007/s00214-022-02893-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02893-7