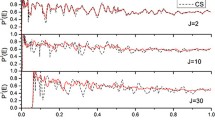
Abstract
Davis’ group and Setser’s group using different experimental techniques measured water vibrational distribution for OH/OD + XH → H2O/HOD + X hydrogen abstraction reactions (X ≡ D, Br, NH2, GeH3). Theoretically, this issue has been studied by several groups: different potential energy surfaces (PESs) have been developed for each system, and quasi-classical trajectory (QCT) and quantum mechanics (QM) calculations have been performed. However, important experimental/theoretical controversies still exist. In the present work, we have revisited and performed new kinetics and dynamics calculations, comparing the theoretical and experimental results on the same footing. In general, theoretical results reproduce reasonably well experimental rate constants and total vibrational energy released to water, ~ 50–60%, although they underestimate water bending excitation. In the present work, we analyse different causes of this theory/experiment discrepancy and propose different mechanisms to explain the water bending excitation for diatom–diatom and polyatomic systems. Finally, we reflect on the ability of QCT and QM calculations to provide reasonable (although not quantitative) predictions for polyatomic reactions and observe that even using full-dimensional QM calculations on very accurate PESs, agreement with the experiment is far from that reached in the case of triatomic systems.












Similar content being viewed by others
References
Strazisar BR, Lin C, Davis HF (2000) Science 290:958
Butkovskaya NI, Setser DW (2003) Int Rev Phys Chem 22:1
Duchovic R, Schatz GC (1986) J Chem Phy. 84:2239
Schatz GC (1988) Comput Phys Commun 51:135
Corchado JC, Espinosa-Garcia J (2009) Phy. Chem Chem Phys 11:10157
Schatz GC, Elgersma H (1980) Chem Phys Lett 73:21
de Ochoa Aspuru G, Clary DC (1988) J Phys Chem A 102:9631
Yang M, Zhang D, Collins M, Lee S (2001) J Chem Phys 115:174
Wu G, Schatz GC, Lendvay G, Fang D, Harding L (2000) J Chem Phys 113:3150
Chen J, Xu X, Xu X, Zhang DH (2013) J Chem Phys 138:154301
Shao K, Chen J, Zhao Z, Zhang DH (2016) J Chem Phys 145:071101
Clary DC, Nyman G, Hernandez R (1994) J Chem Phys 101:3704
Nizamov B, Setser DW, Wang HB, Peslherbe GH, Hase WL (1996) J Chem Phys 105:9897
Liu JY, Li ZS, Dai ZW, Huang XR, Sun CC (2001) J Phys Chem A 105:7707
Oliveira-Filho AGS, Ornellas FR, Bowman JM (2014) J Phys Chem Lett 5:706
Monge-Palacios M, Rangel C, Espinosa-Garcia J (2013) J Chem Phys 138:084305
Monge-Palacios M, Corchado JC, Espinosa-Garcia J (2013) J Chem Phys 138:214306
Espinosa-Garcia J, Corchado JC, Butkovskaya NI, Setser DW (2019) Theor Chem Acc 138:119
Espinosa-Garcia J, Rangel C, Corchado JC (2016) Phys Chem Chem Phys 18:16941
Olsson MHM, Mavri J, Warshel A (2006) Phil Trans R Soc B 361:1417
Duff JW, Truhlar DG (1975) J Chem Phys 62:2477
Gray JC, Truhlar DG, Clemens L, Duff JW, Chapman FM, Morrell GO, Hayes EF (1978) J Chem Phys 69:240
Hu X, Hase WL, Pirraglia Y (1991) J Comput Chem 2:1014
Hase WL, Duchovic RJ, Hu X, Komornicki A, Lim KF, Lu D-H, Peslherbe GH, Swamy KN, Van de Linde SR, Varandas AJC et al (1996) QCPE Bull 16:43
Czakó G, Bowman JM (2009) J Chem Phys 131:244302
Bonnet L, Espinosa-Garcia J (2010) J Chem Phys 133:64108
Czakó G (2012) J Phys Chem A 116:7467
Espinosa-Garcia J, Corchado JC (2017) Phys Chem Chem Phys 19:1580
Hofacker GL, Levine RD (1971) Chem Phys Lett 9:617
Miller WH, Handy NC, Adams JE (1980) J Chem Phys 72:99
Zheng J, Zhang S, Lynch BJ, Corchado JC, Chuang Y-Y, Fast PL, Hu W-P, Liu Y-P, Lynch GC, Nguyen KA, Truhlar DG (2010) POLYRATE-2010-A. University of Minnesota, Minneapolis
Garrett BC, Truhlar DG (1979) J Am Chem Soc 101:4534
Truhlar DG, Isaacson AD, Garrett BC (1985) Generalized transition state theory. In: Baer M (ed) The theory of chemical reactions. CRC, Boca Raton
JANAF Thermochemical Tables, 3rd ed.; Chase MW Jr, Davies CA, Downey JR, Frurip DJ, McDonald RA, Syverud AN Eds.; (1985) National Bureau of Standards: Washington, Vol. 14
Welsch R (2018) J Chem Phys 148:204304
Mielke SL, Allison TC, Truhlar DG, Shwenke DW (1996) J Phys Chem 100:13588
Liu Y-P, Lu DH, Gonzalez-Lafont A, Truhlar DG, Garrett BC (1993) J Am Chem So. 115:7806
Mielke SL, Garret BC, Fleming DG, Truhlar DG (2015) Mol Phys 113:160
Gonzalez-Lavado E, Corchado JC, Suleimanov YV, Green WH, Espinosa-Garcia J (2014) J Phys Chem A 118:3243
Suleimanov YV, Espinosa-Garcia J (2016) J Phys Chem B 120:1418
Espinosa-Garcia J, Rangel C, Suleimanov YV (2017) Phys Chem Chem Phys 19:19341
Monge-Palacios M, Yang M, Espinosa-Garcıa J (2012) Phys Chem Chem Phys 14:4824
Zhang W, Kawamata H, Liu K (2009) Science 325:303
Yang J, Zhang D, Jiang B, Dai D, Wu G, Zhang D, Yang X (2014) J Phys Chem Lett 5:1790
Yang J, Zhang D, Chen Z, Blauert F, Jiang B, Dai D, Wu G, Zhang D, Yang X (2015) J Chem Phys 143:044316
Espinosa-Garcia J, Bravo JL (2008) J Phys Chem A 112:6059
Espinosa-Garcia J (2008) Chem Phys Lett 454:158
Czakó G, Bowman JM (2009) J Am Chem Soc 131:17534
Czakó G, Bowman JM (2011) Phys Chem Chem Phys 13:8306
Palma J, Manthe U (2015) J Phys Chem A 119:12209
Espinosa-Garcia J (2016) J Phys Chem A 120:5
Sun P, Zhang Z, Chen J, Liu S, Zhang DH (2018) J Chem Phys 149:064303
Castillo JF, Suleimanov YV (2017) Phys Chem Chem Phys 19:29170
Wang Y, Li Y, Wang D (2017) Sci Rep 7:40314
NIST Chemical Kinetics Database web page. Standard references database 17, Version 7.0 (Web Version), Release 1.6.8. data version 2015.9
Bonnet L, Espinosa-García J, Corchado JC, Liu S, Zhang DH (2011) Chem Phys Lett 516:137
Liu S, Xiao Ch, Wang T, Chen J, Yang T, Xu X, Zhang DH, Yang X (2012) Faraday Discuss 157:101
Acknowledgments
This work was partially supported by Junta de Extremadura and European Regional Development Fund, Spain (Projects No. GR18010 and IB16013). The authors thank Dr. de Oliveira-Filho and Prof. Bowman for sending us the PES of the OH + HBr reaction, Dr. Liu and Prof. Zhang for sending us the FI-NN PES of the OH + H2 reaction and Prof. Butkovskaya for sharing the revised experimental results of the OH/OD + HBr reactions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Espinosa-Garcia, J., García-Chamorro, M. & Corchado, J.C. Rethinking the description of water product in polyatomic OH/OD + XH (X ≡ D, Br, NH2 and GeH3) reactions: theory/experimental comparison. Theor Chem Acc 139, 63 (2020). https://doi.org/10.1007/s00214-020-2577-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-2577-0