Abstract
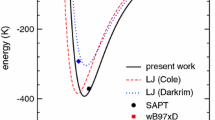
Details concerning the energetics and structure of the ion pair in gaseous chloroethane, obtained at the multi-reference configuration interaction with singles and doubles (MR-CISD) level, are given. It is formed in the third excited state (31A′), and it can be classified as a hydrogen-bonded contact ion pair, although from its total binding energy of 3.28 eV (including extensivity corrections, at the MR-CISD + Q level with the aug-cc-pVTZ basis set, and including zero-point energy corrections) only 0.14 eV is due to an underlying hydrogen bond. It is a highly polar structure, with a dipole moment of 9.56 D. As compared to previous systems for which the same type of bond has been observed, it has a much lower hydrogen-bond energy and a much larger distance between the charge centers. The three lowest frequency vibrational modes of the HBCIP correspond to intermolecular cation–anion modes. The structure here obtained brings important structural questions for HCFCs derived from chloroethane.


Similar content being viewed by others
References
Rossberg M, Lendle W, Pfleiderer G, Tögel A, Eberhard-Ludwig Dreher, Langer E, Rassaerts H, Strack H, Cook R, Beck U, Karl-August Lipper, Loser E, Beutel KK, Mann T (2006) Ullmanns’ Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim, p 26
Rasmussen PP, Shockley JC, Hargadine DA (1994) Hydrogeology and water-quality conditions at the city of Olathe Landfill, East-Central Kansas, 1990-93. In: U. S. geological survey. Water-Resources Investigations Report 94-4166, Lawrence, Kansas
Tschikardt M (2013) The MAK-Collection Part III. Air Monitoring Methods DFG, Deutsche Forschungsgemeinschaft, Wiley-VCH Verlag GmbH & Co, KGaA, p 2
Public Health Statement, Chloroethane. Agency for Toxic Substances and Disease Registry. December 1998. https://www.atsdr.cdc.gov/ToxProfiles/tp105-c1-b.pdf
Miller M, Scully C (2015) Mosby’s textbook of dental nursing, 2nd edn. Elsevier, Edinburgh
Callahan MA, Simak MW, Gabel NW, May IP, Fowler CF, Freed JR, Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, Gould C (1979) Water-related environmental fate of 129 priority pollutants, vol II, halogenated aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics, phthalate esters, polycyclic aromatic hydrocarbons, nitrosamines, and miscellaneous compounds. Office of Water Planning and Standards Office of Water and Waste Management. U. S. Environmental Protection Agency. Washington D. C. 20460, pp 42–43
Calm JM, Hourahan GC (2001) Refrigerant data summary. engineered systems 18(11):74 − 88. https://www.usgbc.org/drupal/legacy/usgbc/docs/LEED_tsac/Calm_Hourahan-Refrigerant_Data-2001.pdf
Wuebbels DJ (2015) Ozone depletion and related topics - ozone depletion potentials. Encyclopedia of atmospheric sciences, vol 4, 2nd edn. Elsevier, Amsterdam
de Medeiros VC, de Andrade RB, Leitão EFV, Ventura E, Bauerfeldt GF, Barbatti M, do Monte SA SA (2016) Photochemistry of CH3Cl: dissociation and CH···Cl hydrogen bond formation. J Am Chem Soc 138:272–280. https://doi.org/10.1021/jacs.5b10573
Rogers NJ, Simpson MJ, Tuckett RP, Dunn KF, Latimer CJ (2010) Vacuum-UV negative photoion spectroscopy of CH3F, CH3Cl and CH3Br. Phys Chem Chem Phys 12:10971–10980. https://doi.org/10.1039/C0CP00234H
Simpson MJ, Tuckett RP (2011) Vacuum-UV negative photoion spectroscopy of gas-phase polyatomic molecules. Int Rev Phys Chem 30:197–273. https://doi.org/10.1080/0144235X.2011.581000
Rodrigues GP, de Lima TML, de Andrade RB, Ventura E, do Monte SA, Barbatti M (2019) Photoinduced formation of H-bonded ion pair in HCFC-133a. J Phys Chem A 123:1953–1961. https://doi.org/10.1021/acs.jpca.8b12482
de Medeiros VC, de Andrade RB, Rodrigues GP, Bauerfeldt GF, Ventura E, Barbatti M, do Monte SA (2018) Photochemistry of CF3Cl: quenching of charged fragments is caused by nonadiabatic effects. J Chem Theor Comput 14:4844–4855. https://doi.org/10.1021/acs.jctc.8b00457
Suto K, Sato Y, Reed CL, Skorokhodov V, Matsumi Y, Kawasaki M (1997) Ion fragment imaging of the ion-pair photodissociation of CH3Cl, CH3Br, C2H5Cl, and C2H5Br at 118 nm. J Phys Chem A 101:1222–1226. https://doi.org/10.1021/jp962883f
Hase WL, Schlegel HB, Balbyshev V, Page M (1996) An ab initio study of the transition state and forward and reverse rate constants for C2H5 ↔ H + C2H4. J Phys Chem 100:5354–5361. https://doi.org/10.1021/jp9528875
Andrei H-S, Solcà N, Dopfer O (2008) IR spectrum of the ethyl cation: evidence for the nonclassical structure. Angew Chem Int Ed 47:395–397. https://doi.org/10.1002/anie.200704163
Klopper W, Kutzelnigg W (1990) MP2-R12 calculations on the relative stability of carbocations. J Phys Chem 94:5625–5630. https://doi.org/10.1021/j100377a040
Hayashi M, Inagusa T (1990) Microwave spectrum, structure and nuclear quadrupole coupling constant tensor of ethyl chloride and vinyl chloride. J Mol Struct 220:103–117. https://doi.org/10.1016/0022-2860(90)80103-Q
de Medeiros VC, do Monte SA, Ventura E (2014) Valence and rydberg states of CH3Cl: a MR-CISD study. RSC Adv 4:64085–64092. https://doi.org/10.1039/C4RA10567B
Rodrigues GP, Ventura E, do Monte SA, Barbatti M (2014) Photochemical deactivation process of HCFC-133a (C2H2F3Cl): a nonadiabatic dynamics study. J Phys Chem A 118:12041–12049. https://doi.org/10.1021/jp507681g
Rodrigues GP, Lucena JR Jr, Ventura E, do Monte AS, Reva I, Fausto R (2013) Matrix isolation infrared spectroscopic and theoretical study of 1,1,1-trifluoro-2-chloroethane (HCFC-133a). J Chem Phys 139:204302–204311. https://doi.org/10.1063/1.4832376
de Medeiros VC, Ventura E, do Monte SA (2012) CASSCF and MR–CISD study of the n − 4 s and n − 4pe Rydberg states of the CF3Cl. Chem Phys Lett 546:30–33. https://doi.org/10.1016/j.cplett.2012.07.037
Lucena JR Jr, Ventura E, do Monte SA, Araújo RCMU, Ramos MN (2007) Dissociation of ground and nσ* states of CF3Cl using multireference configuration interaction with singles and doubles and with multireference average quadratic coupled cluster extensivity corrections. J Chem Phys 127:164320–164331. https://doi.org/10.1063/1.2800020
Shepard R, Lischka H, Szalay PG, Kovar T, Ernzerhof M (1992) A general multireference configuration-interaction gradient program. J Chem. Phys 96:2085–2098. https://doi.org/10.1063/1.462060
Shepard R (1995) The analytic gradient method for configuration interaction wave functions. Modern Electron Struct Theory 2:345–458. https://doi.org/10.1142/9789812832108_0007
Shepard R (1987) Geometrical energy derivative evaluation with MRCI wave functions. Int J Quantum Chem 31:33–34. https://doi.org/10.1002/qua.560310105
Lischka H, Dallos M, Shepard R (2002) Analytic MRCI gradient for excited states: formalism and application to the n-π*, valence and n-(3 s,3p) Rydberg States of Formaldehyde. Mol Phys 100:1647–1658. https://doi.org/10.1080/00268970210155121
Shepard R, Shavitt I, Pitzer RM, Comeau DC, Pepper M, Lischka H, Szalay PG, Ahlrichs R, Brown FB, Zhao J (1988) A progress report on the status of the COLUMBUS MRCI program system. Int J Quantum Chem 34:149–165. https://doi.org/10.1002/qua.560340819
Lischka, H, Shepard R, Shavitt I, Pitzer RM, Dallos M, Müller T, Szalay PG, Brown FB, Ahlrichs R, Böhm HJ, et al. (2019) COLUMBUS, an Ab Initio electronic structure program, release 7.0. www.univie.ac.at/columbus. Accessed November 30, 2019
Lischka H, Shepard R, Pitzer RM, Shavitt I, Dallos M, Müller T, Szalay PG, Seth M, Kedziora GS, Yabushita S et al (2001) New high-level multireference methods in the quantum-chemistry program system COLUMBUS: analytic MR-CISD and MR-AQCC gradients and MR-AQCC-LRT for excited states, GUGA spin-orbit CI, and parallel CI density. Phys Chem Chem Phys 3:664–673. https://doi.org/10.1039/B008063M
Lischka H, Shepard R, Brown FB, Shavitt I (1981) New implementation of the graphical unitary-group approach for multi-reference direct configuration-interaction calculations. Int J Quantum Chem 20:91–100. https://doi.org/10.1002/qua.560200810
Helgaker T, Jensen HA, Jørgensen P, Olsen J, Ruud K, Ågren H, Andersen T, Bak KL, Bakken V, Christiansen O, et al. DALTON, an ab initio electronic structure program, Release 1.0. 1997
Langhoff SR, Davidson ER (1974) Configuration interaction calculations on the nitrogen molecule. Int J Quantum Chem 8:61–72. https://doi.org/10.1002/qua.560080106
Bruna PJ, Peyerimhoff SD, Buenker RJ (1980) The ground state of the CN + ion: a multi-reference CI study. Chem Phys Lett 72:278–284. https://doi.org/10.1016/0009-2614(80)80291-0
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Kendall RA, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806. https://doi.org/10.1063/1.462569
Woon DE, Dunning TH Jr (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371. https://doi.org/10.1063/1.464303
Woon DE, Dunning TH Jr (1994) Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties J Chem Phys 100:2975–2988. https://doi.org/10.1063/1.466439
Hubrich C, Stuhl F (1980) The ultraviolet absorption of some halogenated methanes and ethanes of atmospheric interest J Photochem 12:93–107. https://doi.org/10.1016/0047-2670(80)85031-3
Jeffrey GA, Saenger W (1991) Hydrogen Bonding in Biological Structures. Springer-Verlag, Berlin Heidelberg, p 18
Hobza P, Havlas Z (2000) Blue-Shifting Hydrogen Bonds. Chem Rev 100:4253–4264. https://doi.org/10.1021/cr990050q
Ricks AM, Douberly GE, Schleyer PVR, Duncan MA (2009) Infrared spectroscopy of protonated ethylene: the nature of proton binding in the non-classical structure. Chem Phys Lett 480:17–20. https://doi.org/10.1016/j.cplett.2009.08.063
Dyke JM, Ellis AR, Keddar N, Morris A (1984) A reinvestigation of the first band in the photoelectron spectrum of the ethyl radical. J Phys Chem 88:2565–2569. https://doi.org/10.1021/j150656a027
Ruscic B, Berkowitz J, Curtiss LA, Pople JA (1989) The ethyl radical: photoionization and theoretical studies. J Chem Phys 91:114–121. https://doi.org/10.1063/1.457497
Dixon DA, Feller D, Peterson KA (1997) Accurate calculations of the electron affinity and ionization potential of the methyl radical. J Phys Chem A 101:9405–9409. https://doi.org/10.1021/jp970964l
Garifullina A, Mahroob A, O’Reilly RJ (2016) A database of hemolytic C-Cl bond dissociation energies obtained by means of W1w theory. Chem Data Collect 3–4:21–25. https://doi.org/10.1016/j.cdc.2016.07.003
Check CE, Faust TO, Bailey JM, Wright BJ, Gilbert TM, Sunderlin LS (2001) Addition of polarization and diffuse functions to the LANL2DZ basis set for P-block elements. J Phys Chem A 105:8111–8116. https://doi.org/10.1021/jp011945l
Acknowledgments
The authors thank the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Project 303884/2018-5 and 423112/2018-0), Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP). They are also grateful to the CESUP/UFRGS for the computational facilities and to Prof. Fernando Ornellas from whom many important Quantum Chemistry lessons have been learned.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Festschrift in honor of Prof. Fernando R. Ornellas” Guest Edited by Adélia Justino Aguiar Aquino, Antonio Gustavo Sampaio de Oliveira Filho & Francisco Bolivar Correto Machado.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ventura, E., do Monte, S.A. Hydrogen-bonded contact ion pair in gaseous chloroethane: a multi-reference configuration interaction with singles and doubles (MR-CISD) study including extensivity corrections. Theor Chem Acc 139, 49 (2020). https://doi.org/10.1007/s00214-020-2561-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-2561-8