Abstract
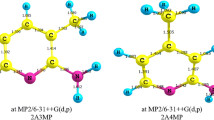
Electronic structure calculations have been performed for the characterization of the different conformers of the herbicide metolachlor (MC), (2-chloro-N-(2-methyl-6-ethylphenyl)-N-(2-methoxy-1-methylethyl) acetamide), the radical structures obtained through the photo chemical breaking of the C–Cl bond and the most important, experimentally identified mono-hydroxylated photo-fragmentation products. Only the S-metolachlor enantiomer that shows herbicide activity was studied. The goal of the study has been twofold. The first target has been the conformational analysis of the neutral molecule and the corresponding radical. Ten different conformers were identified for the S-metolachlor enantiomer, all of them exhibiting a near perpendicular disposition of the amidyl and the phenyl planes. They were classified into two families of minima depending on the orientation of the carbonyl group (either cis or trans with respect to the phenyl ring). All the minima in the S-cis family were found to lie higher than those in the S-trans family. The free energy of isomerization between the lowest minima of each family was found to be \( \Delta G_{298}^{0} \) = 6.2 kcal/mol, while the isomerization barrier around the amide C–N bond causing atropisomerism was calculated to be 34.9 kcal/mol (in good agreement with the experimental energy of activation, 36.9 ± 3 kcal/mol) [45]. The second part of the work is devoted to the computational characterization of the most important isomeric forms of the metolachlor radical and the major photodegradation mono-hydroxylated metabolites formed through the coupling of the metolachlor radical with OH. Reaction enthalpies and free energies for the various hydroxylation pathways have been calculated. Based on their order, the relative significance of the various hydroxylation channels is justified and the preference in the phenyl ring hydroxylation is established. The lowest exothermicity value determined, − 5.8 kcal/mol at the M06/6-31 + G(d,p) level, corresponds to phenyl group hydroxylation at the p-position with regard to C–N bond, in excellent agreement with the experimental findings.








Similar content being viewed by others
References
Barbash JE, Thelin GP, Kolpin DW, Gilliom R (1999) Distribution of major herbicides in ground water of the United States. U.S. Geological Survey, Water-Resources Investigations Report 98-4245, Sacramento, CA
United States Environmental Protection Agency (2017) Pesticides. https://www.epa.gov/pesticides
Burrows HD, L Canle M, Santaballa JA, Steenken S (2002) Reaction pathways and Photodegradation of pesticides. J Photochem Photobiol B Biol 67:71–108
Reade JPH, Cobb AH (2002) Herbicides: mode of action and metabolism. In: Naylor REL (ed) Weed management handbook. Blackwell Science, Oxford
Böger P (2003) Mode of action of chloroacetamides and functionally related compounds. J Pest Sci 28:324–329
Benitez FJ, Acero JL, Real FJ, Maya C (2004) Modeling of photooxidation of acetamide herbicides in natural waters by UV radiation and the combinations UV/H2O2 and UV/O3. J Chem Technol Biotechnol 79:987–997
Carlson DL, Than KD, Roberts AL (2006) Acid and base-catalyzed hydrolysis of chloroacetamide herbicides. J Agric Food Chem 54:4740–4750
Chesters G, Simsiman GV, Levy J, Alhajjar BI, Riyadh N, Fathulla RN, Harkin JM (1989) Environmental fate of alachlor and metolachlor. Rev Environ Contamin Toxicol 110:1–74
Aubertot JN, Barbier JM, Carpentier A, Gril JN, Guichard L, Lucas P, Savary S, Voltz M (2005) Pesticides, agriculture et environnement. Réduire l’ utilisation des pesticides et en limiter les impacts environnementaux. Rapport d’Expertise scientifique collective Inra-Cemagref
Li Z, Jennings A (2017) Worldwide regulations of standard values of pesticides for human health risk control: a Review. Int J Environ Res Public Health 14:826–830
Aga DS, Heberle S, Rentsch D, Hany R, Müller SR (1999) Sulfonic and oxanilic acid metabolites of acetanilide herbicides: separation of diastereomers and enantiomers by capillary zone electrophoresis and identification by 1H NMR Spectroscopy. Environ Sci Technol 33:3462–3468
Kant Kabler A, Chen S (2006) Determination of the 1’S and 1’R diastereomers of metolachlor and S-metolachlor in water by chiral liquid chromatography-mass spectrometry (LC/MS/Ms). J Agric Food Chem 54:6153–6158
Klein C, Schneider RJ, Meyer MT, Aga DS (2006) Enantiomeric separation of metolachlor and its metabolites using LC–MS and CZE. Chemosphere 62:1591–1599
Aboul Eish MYZ, Wells MJM (2008) Monitoring stereoselective degradation of metolachlor in a constructed wetland: use of statistically valid enantiomeric and diastereomeric fractions as opposed to ratios. J Chromate Sci 46:269–275
Ng HYF, Gaynor JD, Tan CS, Drury CF (1995) Dissipation and loss of atrazine and metolachlor in surface and subsurface drain water: a case study. Wat Res 29:2309–2317
O’Connell PJ, Harms CT, Allen JRF (1998) Metolachlor, S-metolachlor and their role within sustainable weed-management. Crop Prot 17:207–212
Fava L, Bottoni P, Crobe A, Funari E (2000) Leaching properties of some degradation products of alachlor and metolachlor. Chemosphere 41:1503–1508
Accinelli C, Dinelli G, Vicari A (2001) Atrazine and metolachlor degradation in subsoils. Biol Fertile Soils 33:495–500
Novak SM, Portal JM, Schiavon M (2001) Effects of soil type upon metolachlor losses in subsurface drainage. Chemosphere 42:235–244
Sanyal D, Kulshrestha G (2002) Metabolism of metolachlor by fungal cultures. J Agric Food Chem 50:499–505
Rice PA, Anderson TA, Coats JR (2002) Degradation and persistence of metolachlor in soil: effects of concentration, soil, moisture, soil depth and sterilization. Environ Toxic Chem 21:2640–2645
Mersie W, McNamee C, Seybold C, Wu J, Tierney D (2004) Degradation of metolachlor in bare and vegetated soils and simulated water-sediment systems. Environ Toxic Chem 23:2627–2632
Caracciolo AB, Giuliano G, Grenni P, Guzzella L, Pozzoni F, Bottoni P, Fava L, Crobe A, Orrù M, Funari E (2005) Degradation and leaching of the herbicide metolachlor and diuron: a case study in the area of Northern Italy. Environ Pollut 134:525–534
White PM Jr, Potter TL (2010) Metolachlor and chlorothalonil dissipation in gypsum amended soil. J Environ Sci Health B 45:728–737
Vryzas Z, Papadakis EN, Oriakli K, Moysiadis TP, Papadopoulou-Mourkidou E (2012) Biotransformation of atrazine and metolachlor within soil profile and changes in microbial communities. Chemosphere 89:1330–1338
Prueger JH, Alfieri J, Gish TJ, Kustas WP, Daughtry CST, Hatfield JL, McKee LG (2017) Multi-year measurements of field-scale metolachlor volatilization. Water Air Soil Pollut 228:84–92
Kochany J, Maguire JR (1994) Sunlight photodegradation of metolachlor in water. J Agric Food Chem 42:406–412
Pignatello JJ, Sun Y (1995) Complete oxidation of metolachlor and methyl parathion in water by the photoassisted fenton reaction. Wat Res 29:1837–1844
Mathew R, Khan SU (1996) Photodegradation of metolachlor in water in the presence of soil mineral and organic constituents. J Agric Food Chem 44:3996–4000
Lin YJ, Karuppiah M, Shaw A, Gupta G (1999) Effect of simulated sunlight on atrazine and metolachlor toxicity of surface waters. Ecotoxixol Environ Saf 43:35–37
Wilson RI, Mabury SA (2000) Photodegradation of metolachlor: isolation, identification and quantification of monochloroacetic acid. J Agric Food Chem 48:944–950
Dinelli G, Accinelli C, Vicari A, Catizone P (2000) Comparison of the persistence of atrazine and metolachlor under field and laboratory conditions. J Agric Food Chem 48:3037–3043
Sakkas VA, Arabatzis IM, Konstantinou IK, Dimou AD, Albanis TA, Falaras P (2004) Metolachlor photocatalytic degradation using TiO2 photocatalysts. Appl Catal B Environ 49:195–205
Dimou AD, Sakkas VA, Albanis TA (2005) Metolachlor photodegradation study in aqueous media under natural and simulated solar irradiation. J Agric Food Chem 53:694–701
Wu C, Shemer H, Linden KG (2007) Photodegradation of metolachlor applying UV and UV/H2O2. J Agric Food Chem 55:4059–4065
Hladik ML, Bouwer EJ, Roberts AL (2008) Neutral degradates of chloroacetamide herbicides: occurrence in drinking water and removal during conventional water treatment. Water Res 42:4905–4914
Goulden PH, Coffinet S, Genty C, Bourcier S, Sablier M, Bouchonnet S (2011) Investigation of the unusual behavior of metolachlor under chemical ionization in a hybrid 3d ion trap mass spectrometer. Anal Chem 83:7587–7590
Bouchonnet S, Kinani S, Souissi Y, Bourcier S, Sablier M, Roche P, Boireau V, Ingrand V (2011) Investigation of the dissociation of metolachlor, acetochlor and alachlor under electron ionization—application to the identification of ozonation products. Rapid Commun Mass Spectrom 25:93–103
Restivo J, Orfao JJM, Armenise S, Garcia-Bordeje E, Pereira MFR (2012) Catalytic ozonation of metolachlor under continuous operation using nanocarbon materials grown on a ceramic monolith. J Hazard Mater 239–240:249–256
Coffinet S, Rifai A, Genty C, Souissi Y, Bourcier S, Sablier M, Bouchonnet S (2012) Characterization of the photodegradation products of metolachlor: structural elucidation, potential toxicity and persistence. J Mass Spectrom 47:1582–1593
Souissi Y, Bouchonnet S, Bourcier S, Kusk KO, Henrik MS, Andersen R (2013) Identification and ecotoxicity of degradation products of chloroacetamide herbicides from UV-treatment of water. Sci Total Environ 458–460:527–634
Otero R, Esquivel D, Ulibarri MA, Romero-Salguero FJ, Van der Voort P, Fernandez JM (2014) Mesoporous phenolic resin and mesoporous carbon for the removal of s-metolachlor and bentazon herbicides. Chem Eng J 251:92–101
Nicol E, Genty C, Bouchonnet S, Bourcier S (2015) Structural elucidation of metolachlor photoproducts by liquid chromatography/high-resolution tandem mass spectrometry. Rapid Commun Mass Spectrom 29:2279–2286
Orge CA, Pereira MFR, Faria JL (2017) Photocatalytic-assisted ozone degradation of metolachlor aqueous solution. Chem Eng J 318:247–253
Moser H, Rihs G, Sauter H (1982) Der Einfluß von Atropisomerie und chiralem Zentrum auf die biologische Aktivität des Metolachlor. Z Naturforsch 37b:451–462
Blaser HU (2002) The chiral switch of (S)-Metolachlor: a personal account of an industrial Odyssey in asymmetric catalysis. Adv Synth Catal 344:17–31
Machinori O (1983) Recent advances in atropisomerism. In: Allinger NL, Eliel EL, Wilen SH (eds) Topics in stereochemistry. Wiley, Hoboken, pp 1–82
Alkorta I, Elguero J, Roussel C, Vanthuyne N, Patrick Piras P (2012) Atropisomerism and axial chirality in heteroaromatic compounds. Adv Heter Chem 105:1–188
Koch W, Holthausen MC (2001) A Chemist’s guide to density functional theory. Wiley, Hoboken
Zhao Y, Truhlar DG (2006) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Walker M, Harvey AJ, Sen A, Dessent CE (2013) Performance of M06, M06-2X and M06-HF density functionals for conformationally flexible atomic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J Phys Chem A 117:12590–12600 (and references therein)
Montgomery JA Jr, Frisch MJ, Ochterski JW, Peterson GA (2000) A complete basis set model chemistry, VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda KR, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Straroverov VN, Kobayashi R, Normand J, Raghavashari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo VC, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc., Wallingford, CT
Pilling MJ (1996) Radical-radical reactions. Ann Rev Phys Chem 47:81–108
Acknowledgements
ONV thanks economic support for this project from Pedeciba and UdelaR.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salta, Z., Kosmas, A.M. & Ventura, O.N. Computational characterization of the herbicide metolachlor and its mono-hydroxylated photodegradation products. Theor Chem Acc 137, 151 (2018). https://doi.org/10.1007/s00214-018-2353-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2353-6