Abstract
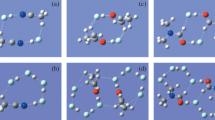
We have performed theoretical studies on sixteen molecular cubes for both (NH3·HCl)(H2O)6 and (NH3·HF)(H2O)6. We use an empirical gauge, based upon the N–H and H–X bond lengths, to categorize the degree to which the cubes are neutral adduct or ion pair in character. On this basis, we describe all sixteen cubes of the former as highly ionized, but only five of the latter as greater than 85% ionic in character. Addition of one or two bridging water molecules to form (NH3·HF)(H2O)7 or (NH3·HF)(H2O)8 raises the percent ionic character to greater than 85% for these systems. The relative energy of the cubes can be categorized based on simple chemical principles. The computed vibrational frequency corresponding to the proton stretch in the N–H–F framework shows the highest degree of redshifting for systems near 50% ion-pair character. Molecular cubes close to neutral adduct or to ion-pair character show less redshifting of this vibrational motion.











Similar content being viewed by others
References
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, New York
Maréchal Y (2007) The hydrogen bond and the water molecule. Elsevier, New York
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Grabowski SJ (ed) (2006) Hydrogen bonding—new insights. Challenges and advances in computational chemistry and physics, vol 3. Springer, New York
Gilli G, Gilli P (2009) The nature of the hydrogen bond: outline of a comprehensive hydrogen bond theory. Oxford University Press, New York
Buckingham AD, Del Bene JE, McDowell SAC (2008) The hydrogen bond. Chem Phys Lett 463:1–10
Skvortsov D, Lee SJ, Choi MY, Vilesov AF (2009) J Phys Chem A 113(26):7360–7365
Gutberlet A, Schwaab G, Özgür B, Masia M, Kaczmarek A, Forbert H, Havenith M, Marx D (2009) Science 324:1545–1548
Re S, Osamura Y, Suzuki Y, Schaefer HF III (1998) Structures and stability. J Chem Phys 109:973–977
Morrison AM, Flynn SD, Liang T, Douberly GE (2010) J Phys Chem A 114(31):8090–8098
Gómez PC, Gálvez O, Escribano R (2009) Phys Chem Chem Phys 11:9710–9719
Anderson KE, Siepmann JI, McMurry PH, VandeVondele J (2008) J Am Chem Soc 130:14144–14147
Osuna S, Swart M, Baerends EJ, Bickelhaupt FM, Solà M (2009) Chem Phys Chem 10:2955–2965
Robertson WH, Johnson MA (2003) In: Leone SR, Alivisatos P, McDermott AE (eds) Annual review of physical chemistry, vol 54. Annual Reviews, Palo Alto, pp 173–213
Markovitch O, Agmon N (2007) J Phys Chem A 111(12):2253–2256
Belair SD, Francisco JS (2003) Phys Rev A 67(6):063206
Gruenloh CJ, Carney JR, Arrington CA, Zwier TS, Fredericks SY, Jordan KD (1997) Science 276:1678–1681
Kuo J-L, Klein ML (2004) J Chem Phys 120:4690–4695
Stob MJ (1994) Private communication, Calvin College, July, 2007, Mathematica application of oriented graph theory. http://www.mathworld.wolfram.com/OrientedGraph. Harary, F. Graph Theory. Addison-Wesley, Reading, p 10
Del Bene JE, Jordan MJT (1998) J Chem Phys 108(8):3205
Cazar RA, Jamka AJ, Tao F-M (1998) J Phys Chem A 102:5117–5123
Snyder JA, Cazar RA, Jamka AJ, Tao F-M (1999) J Phys Chem A 103:7719–7724
Li R-J, Li Z-R, Wu D, Hao X-Y, Li Y, Wang B-Q, Tao F-M, Sun CC (2003) Chem Phys Lett 372:893–898
DeKock RL, Schipper LA, Dykhouse SC, Heeringa LP, Brandsen BM (2009) J Chem Educ 86(12):1459–1464
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1988) Phys Rev A 38:3098–3100
Lenz A, Ojamae L (2005) Phys Chem Chem Phys 7:1905–1911
Koch W, Holthausen MC (2001) A chemist’s guide to density functional theory, 2nd edn. Wiley, New York, pp 213–232
Lee HM, Suh SB, Lee JY, Tarakeshwar P, Kim KS (2000) J Chem Phys 112:9759–9772
Silica MC, Muñoz-Caro C, Niño A (2005) ChemPhysChem 6:139–147
Lozynski M, Rusinska-Roszak D, Mack HJ (1998) J Phys Chem A 102:2899–2903
Odde S, Mhin BJ, Lee KH, Tarakeshwar P, Kim KS (2006) J Phys Chem A 110:7918–7924
Boese AD, Martin JML, Klopper WJ (2007) J Phys Chem A 111:11122–11133
Woon DE, Dunning TH Jr (1993) J Chem Phys 98:1358–1371
Christie RA, Jordan KD (2001) J Phys Chem A 105:7551–7558
Xantheas SS (2005) J Am Chem Soc 117:10373–10380
Xantheas SS, Dunning TH Jr (1993) J Chem Phys 99:8774–8792
Xantheas SS (1994) J Chem Phys 100:7523–7534
Perdew JP (1986) Phys Rev B 33:8822–8824
Adamo C, Barone V (1998) J Chem Phys 108(2):664
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Peterson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Slavador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03. Revision E.02 edn. Gaussian, Inc., Wallingford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JJA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Revision B.01 edn. Gaussian, Inc., Wallingford
Song J-W, Tsuneda T, Sato T, Hirao K (2011) Theor Chem Acc. http://www.dx.doi.org/10.1007/s00214-011-0997-6
Saito T, Nishihara S, Yamanaka S, Kitagawa Y, Kawakami T, Yamada S, Isobe H, Okumura M, Yamaguchi K (2011) Theor Chem Acc. http://www.dx.doi.org/10.1007/s00214-011-0914-z
Radhakrishnan TP, Herndon WC (1991) Graph theoretical analysis of water clusters. J Phys Chem 95(26):10609–10617
McDonald S, Ojamäe L, Singer SJ (1998) J Phys Chem A 102(17):2824–2832
Biczysko M, Latajka Z (1999) Chem Phys Lett 313(1–2):366–373
Barnes AJ, Latajka Z, Biczysko M (2002) J Mol Struct 614(1–3):11–21
Abkowicz-Bienko A, Biczysko M, Latajka Z (2000) Comput Chem 24(3–4):303–309
Li R-J, Li Z-R, Wu D, Chen W, Li Y, Wang B-Q, Sun C–C (2005) J Phys Chem A 109(4):629–634
Cheung JT, Dixon DA, Herschbach DR (1988) J Phys Chem 92(9):2536–2541
Blondel C, Delsart C, Goldfarb F (2001) J Phys B Atomic Mol Optical Phys 34(9):L281–L288
Berzinsh U, Gustafsson M, Hanstorp D, Klinkmüller A, Ljungblad U, Mårtensson-Pendrill AM (1995) Phys Rev A 51(1):231–238
Di Lonardo G, Douglas AE (1973) Can J Phys 51(4):434–445
Michel M, Korolkov MV, Weitzel K-M (2002) Phys Chem Chem Phys 4(17):4083–4086
Hunter EPL, Lias SG (1998) J Phys Chem Ref Data 27(3):413
Solimannejad M, Scheiner S (2007) J Phys Chem A 111:4431–4435
Gardenier GH, Johnson MA, McCoy AB (2009) J Phys Chem A 113(16):4772–4779
Soloveichik PO, Donnell BA, Lester MI, Francisco JS, McCoy AB (2010) J Phys Chem A 114(3):1529–1538
Dixon DA, Feller D, Peterson KA (2001) J Chem Phys 115(6):2576
Acknowledgments
We gratefully acknowledge the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research. We thank Laura A. Schipper and Stephanie C. Dykhouse for the preliminary work that they performed on this project. Computer hardware was provided by a Major Research Instrumentation Grant from the National Science Foundation, Award No. OCI-0722819.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Akira Imamura on the occasion of his 77th birthday and published as part of the Imamura Festschrift Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
1.1 Empirical gauge of hydrogen bond versus ion-pair structure
We define a “reference” bond length and examine the relative difference between the reference bond length and that found in the molecule at hand. We use NH3·HF and NH3·HCl as our example molecules and employ our theoretical results to illustrate the method. Our empirical gauge relates the bond lengths \( {\text{X}}\overset {r_{1} } \longleftrightarrow {\text{H}}\overset {r_{2} } \longleftrightarrow {\text{N}} \) to reference bond lengths. It is the distortion between the actual bond length and a reference bond length for each bond that measures the extent to which the structure is classified as a hydrogen bond adduct versus an ion-pair structure. In the following paragraph, we employ the aug-cc-pVDZ set for our theoretical results. Where experimental work is known, we place that in parentheses directly after the theoretical value.
The free molecule HF has a bond length of 0.926 Å (0.917 Å), and the corresponding value for HCl is 1.295 Å (1.274 Å). These computed values are labeled r 1ref for HF and HCl, respectively. The second reference, r 2ref, is the N–H bond length in NH4 +; we chose its value to be 1.0217 Å, [60].
We then define the following quantities that refer to ion-pair character and hydrogen bond character.
The experimental structure of NH3·HF has not been reported, but the theoretical results are r 1 = 0.966 Å and r 2 = 1.662 Å. The corresponding theoretical values for NH3·HCl are r 1 = 1.370 Å and r 2 = 1.668 Å. From these results, we obtain i-p character = 0.043 and h-b character = 0.583 for NH3·HF and obtain i-p character = 0.058 and h-b character = 0.589 for NH3·HCl. In order to convert these into a percent contribution of each structure, we divide by a normalization factor, N, defined as the sum of these quantities.
The percent ion-pair character and percent hydrogen bond character for a given molecular structure are defined as follows:
We obtain 93% h-b, 7% i-p for NH3·HF and 91% h-b, 9% i-p for NH3·HCl. These results provide a quantitative way to report the amount of distortion that the acid undergoes upon bonding to the base. They confirm that the hydrogen bond is overwhelmingly an adduct pair, and not an ion pair. They show that NH3·HCl is more “ionic” than NH3·HF, which makes qualitative sense, since chemists have many reasons to refer to HF as a weaker acid than HCl.
The empirical formula that we utilize here provides results that allow a quick empirical look at the nature of the molecular adduct. Scheiner [1] provided an alternative view based upon a parameter he called ρ defined as ρ = Δr(XH) − Δr(NH).
Rights and permissions
About this article
Cite this article
DeKock, R.L., Brandsen, B.M. & Strikwerda, J.R. Theoretical study of formation of ion pairs in (NH3·HCl)(H2O)6 and (NH3·HF)(H2O)6 . Theor Chem Acc 130, 871–881 (2011). https://doi.org/10.1007/s00214-011-1032-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-011-1032-7