Abstract
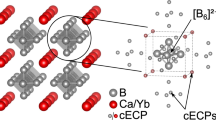
Equatorial/apical bond repartitioning in TBP clusters, formed by main group elements only (e.g., B5H5 2−, B3H3N2, etc.) or mixed with metal atoms (e.g., [(L2M)3S2]n, [(L3M)3S2]n, [(CpM)3S2]n), is critically analyzed from the horizontal comparison of the experimental structures and in terms of basic MO concepts. The ideas are double-checked through specific DFT calculations or existing ab initio results. Based on the Wade’s rules for closo-clusters, the five vertices systems are normally characterized by six skeletal electron pairs. In the main group clusters, the electron delocalization at the equatorial edges depends on the electronegativity of the apical groups and for the Beq–Beq bonds an inverse relationship between bond strength and bond length is remarked. In [(L2M)3S2]n compounds with a total electron count (TEC) of 48, the six bonding electron pairs localize at the apical M–S bonds. In [(L3M)3S2]n or [(CpM)3S2]n, also with TEC = 48, the effective atomic number rule predicts three single M–M single bonds besides the six M–S apical ones. In actuality, only partial M–M bonding can be considered due to the intermediation of the capping sulphur atoms, that help shifting the antibonding character of populated radial levels with that of the vacant tangential ones (bonding). The qualitative MO arguments are supported by the topological nature of the calculated DFT wave functions. Moreover, the MO nature of three lowest LUMOs for 48e− species help to rationalize experimental structural trends observed for the addition of up to five electrons.










Similar content being viewed by others
Notes
Cambridge Structural Database System, Cambridge Crystallographic data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK.
The EAN rule predicts that the number of M–M bonds (n) is equal to the half difference between the highest electron configuration achievable by all the metals (in this case 3 × 18) and the actual number of valence electrons (=TEC). For 48e− TBP compounds, n = 3.
To justify some experimental data it has been assumed that a2′ may even be lower than 2e′ [55].
References
Bencini A (2008) Inorg Chim Acta 361:3820. doi:10.1016/j.ica.2008.03.076
Hoffmann R (1998) Theochem 424:1. doi:10.1016/S0166-1280(97)00219-4
Improta R, Barone V, Santoro F (2007) Angew Chem Int Ed 46:405–408. doi:10.1002/anie.200602907
Barone V, Improta R, Rega N (2008) Acc Chem Res 41:605. doi:10.1021/ar7002144
Colle R, Fortunelli A, Re N, Salvetti O (1988) J Am Chem Soc 110:8016. doi:10.1021/ja00232a010
ADF2008 01; SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam. Available at http://www.scm.com
Te Velde G, Bickelhaupt FM, van Gisbergen SJA, Fonseca Guerra C, Baerends EJ, Snijders JG, Ziegler TJ (2001) Comput Chem 22:931
Fonseca Guerra C, Snijders JG, Te Velde G, Baerends EJ (1998) Theor Chem Acc 99:391
Mealli C, Proserpio DM (1990) J Chem Ed 67:399
Mealli C, Ienco A, Proserpio DM (1998) Book of Abstracts of the XXXIII ICCC, ICCC, Florence, Italy, p 510
Li H, Carpenter GB, Sweigart DA (2000) Organometallics 19:1823. doi:10.1021/om000100d
Ienco A, Caporali M, Zanobini F, Mealli C (2009) Inorg Chem 48 (in press). doi:10.1021/ic8023748
Wade K (1971) J Chem Soc Chem Commun 792
Wade K (1976) Adv Inorg Radiochem 18:1
O’Neill ME, Wade K (1982) Comprehensive organometallic chemistry. In: Wilkinson G, Stone FGA, Abel E (eds) Pergamon Press, New York
Brown EC, York TJ, Antholine WE, Ruiz E, Alvarez S, Tolman WB (2005) J Am Chem Soc 127:13752. doi:10.1021/ja053971t
York JT, Bar-Nahum I, Tolman WB (2007) Inorg Chem 46:8105. doi:10.1021/ic700760p
Mealli C, Ienco A, Poduska A, Hoffmann R (2008) Angew Chem Int Ed 47:2864. doi:10.1002/anie.200705296
Isobe K, Ozawa Y, Vázquez de Miguel A, Zhu TW, Zhao KM, Nishioka T, Ogura T, Kitagawa T (1994) Angew Chem Int Ed Engl 33:1882. doi:10.1002/anie.199418821
Nishioka T, Kitayama H, Breedlove BK, Shiomi K, Kinoshita I, Isobe K (2004) Inorg Chem 43:5688. doi:10.1021/ic049855t
Poduska A, Hoffmann R, Ienco A, Mealli C (2009) Chem Asian J 4:302. doi:10.1002/asia.200800333
Mealli C, Hoffmann R, Alvarez S (2009) Submitted for publication
von R, Schleyer P, Subramanian G, Dransfeld A (1996) J Am Chem Soc 118:9988. doi:10.1021/ja962036q
Burdett JK, Eisenstein O (1995) J Am Chem Soc 117:939
Dixon DA, Klier DA, Halgreen TA, Hall JH, Lipscomb WN (1977) J Am Chem Soc 99:7834. doi:10.1021/ja00466a014
Graham GD, Marynick DS, Lipscomb WN (1980) J Am Chem Soc 102:2939. doi:10.1021/ja00529a012
King RB, Rouvray DH (1977) J Am Chem Soc 99:7834. doi:10.1021/ja00466a014
Aihara J (1978) J Am Chem Soc 100:3339. doi:10.1021/ja00479a015
Jemmis ED, Subramanian GJ (1994) J Phys Chem 98:9222. doi:10.1021/j100088a022
Jemmis ED, Jayasree EG (2003) Acc Chem Res 36:816. doi:10.1021/ar0300266
von R Schleyer P, Najafian K (1998) Inorg Chem 37:3454. doi:10.1021/ic980110v
Sahin Y, Präsang C, Hofmann M, Geiuseler G, Massa W, Berndt A (2005) Angew Chem Int Ed 44:1643. doi:10.1002/anie.200462397
Antipin M, Boese R, Bläser D, Maulitz A (1997) J Am Chem Soc 119:326. doi:10.1021/ja961884i
Mealli C, Costanzo F, Ienco A, Peruzzini M, Perez-Carreño E (1998) Inorg Chim Acta 275:366. doi:10.1016/S0020-1693(97)06066-0
Hoffmann R (1982) Angew Chem Int Ed Engl 21:711. doi:10.1002/anie.198207113
Phillips AD, Ienco A, Reinhold J, Böttcher H-C, Mealli C (2006) Chem Eur J 12:4691. doi:10.1002/chem.200501071
Cecconi F, Ghilardi CA, Midollini S, Orlandini A (1991) Z Naturforsch B 46:1161
Cecconi F, Ghilardi CA, Midollini S, Orlandini A, Vacca A, Ramírez JA (1990) J Chem Soc Dalton Trans 773
Fenske D, Fleischer H, Krautscheid H (1990) Z Naturforsch B 45:127
Ghilardi CA, Midollini S, Orlandini A, Scapacci G (1992) J Chem Soc Dalton Trans 2909
Haiduc I, Semeniuc RF, Campian M, Kravtsov VC, Simonov YA (2003) Polyhedron 22:2895
Matsumoto K, Saiga N, Tanaka S, Ooi S (1991) J Chem Soc Dalton Trans 1265
Vicic DA, Jones WD (1999) J Am Chem Soc 121:7606
Galli D, Garlaschelli L, Ciani G, Fumagalli A, Martinengo S, Sironi A (1984) J Chem Soc 55
Della Pergola R, Garlaschelli L, Martinengro S, Demartin F, Manassero M, Sansoni M (1986) J Chem Soc Dalton Trans 2463. doi:10.1039/dt9860002463
Casado M, Perez-Torrente JJ, Ciriano MA, Edwards AJ, Lahoz FJ, Oro LA (1999) Organometallics 18:5299. doi:10.1021/om990623p
Fang Z-G, Hor TSA, Mok KF, Ng S-C, Liu L-K, Wen Y-S (1993) Organometallics 12:1009. doi:10.1021/om00028a010
Yao W-R, Guo D-S, Liu ZH, Zhang Q-F (2003) J Mol Struct 657:165. doi:10.1016/S0022-2860(03)00415-0
Huang K-C, Tsai Y-C, Lee G-H, Peng S-M, Shieh M (1997) Inorg Chem 36:4421. doi:10.1021/ic961482b
Seidel R, Schnautz B, Henkel G (1996) Angew Chem Int Ed Engl 35:1710. doi:10.1002/anie.199617101
Zhuang B, Chen J, He L, Chen J, Zhou Z, Wu K (2004) J Organomet Chem 689:2674. doi:10.1016/j.jorganchem.2004.05.036
Pulliam CR, Thoden JB, Stacy AM, Spencer B, Englert MH, Dahl LF (1991) J Am Chem Soc 113:7398. doi:10.1021/ja00019a041
Nishioka T, Isobe K (1994) Chem Lett 1661. doi:10.1246/cl.1994.1661
Zimmermann C, Anson CE, Eckermann AL, Wunder M, Fischer G, Keilhauer I, Herrling E, Pilawa B, Hampe O, Weigend F, Dehnen S (2004) Inorg Chem 43:4595. doi:10.1021/ic034876t
North TE, Thoden JB, Spencer B, Dahl LF (1993) Organometallics 12:1299. doi:10.1021/om00028a053
Carrasco R, Aullòn G, Alvarez S (2009) Chem Eur J 15:536. doi:10.1002/chem.200800914
Wiberg KB, Walker FH (1982) J Am Chem Soc 104:5239. doi:10.1021/ja00383a046
Nied D, Klopper WF, Breher A (2009) Angew Chem Int Ed 47:2864
Mealli C, Ienco A (to be published)
Rives AB, You X-Z, Fenske RF (1982) Inorg Chem 21:2286. doi:10.1021/ic00136a032
Maj JJ, Rae AD, Dahl LF (1982) J Am Chem Soc 104:3054. doi:10.1021/ja00375a019
Dehnen S (2005) Z Anorg Allg Chem 631:604. doi:10.1002/zaac.200400512
Zimmermann C, Anson CE, Dehnen S (2007) J Cluster Sci 18:618. doi:10.1007/s10876-007-0130-0
Frisch MJ et al (2004) Gaussian 03, revision C.02. Gaussian Inc., Wallingford, CT
Becke AD (1993) J Chem Phys 98:5648. doi:10.1063/1.464913
Lee C, Yang W, Parr R (1998) Phys Rev B 37:785. doi:10.1103/PhysRevB.37.785
Dolg M, Stoll H, Preuss H, Pitzer RM (1993) J Phys Chem 97:5852. doi:10.1021/j100124a012
Hariharan PC, Pople JA (1973) Theor Chim Acta 28:213. doi:10.1007/BF00533485
Acknowledgments
Thanks are expressed to Professor Roald Hoffmann for comments and suggestions. The work was carried out under the Project No. 7 of the DPM at CNR. Computations have performed thanks to the time allotted by CINECA and CASPUR under the agreement with CNR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor Oriano Salvetti and published as part of the Salvetti Memorial Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mealli, C., Messaoudi, A. & Ienco, A. An overview of the electronic structure in trigonal bipyramidal clusters of main elements or mixed with transition metals. Theor Chem Acc 123, 365–373 (2009). https://doi.org/10.1007/s00214-009-0563-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0563-7