Abstract
Rationale
The gut microbiota may play an important role in the development and functioning of the mammalian central nervous system. The assumption of the experiment was to prove that the use of probiotic bacterial strains in the diet of mice modifies the expression of brain proteins involved in metabolic and immunological processes.
Objectives and results
Albino Swiss mice were administered with Bifidobacterium longum Rosell®-175 or Lactobacillus rhamnosus JB-1 every 24 h for 28 days. Protein maps were prepared from hippocampal homogenates of euthanized mice. Selected proteins that were statistically significant were purified and concentrated and identified using MALDI-TOF mass spectrometry. Among the analysed samples, 13 proteins were identified. The mean volumes of calcyon, secreted frizzled-associated protein 3, and catalase in the hippocampus of mice from both experimental groups were statistically significantly higher than in the control group. In mice supplemented with Lactobacillus rhamnosus JB-1, a lower mean volume of fragrance binding protein 2, shadow of prion protein, and glycine receptor α4 subunit was observed compared to the control.
Conclusion
The psychobiotics Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1enhances expression of proteins involved in the activation and maturation of nerve cells, as well as myelination and homeostatic regulation of neurogenesis in mice. The tested psychobiotics cause a decrease in the expression of proteins associated with CNS development and in synaptic transmission, thereby reducing the capacity for communication between nerve cells. The results of the study indicate that psychobiotic bacteria can be used in auxiliary treatment of neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Widespread chemical treatment of infectious and non-infectious diseases, poor diet, and numerous stress factors are attributes of the contemporary fast pace of human life (Cohen et al. 2015), as well as industrial livestock farming focused on attaining the highest possible production (Graham et al. 2008; Manyi-Loh et al. 2018; Budreviciute et al. 2020). A common phenomenon in these conditions, observed in both humans and animals, is functional disorders of the gastrointestinal tract (GIT), whose functioning largely depends on the intestinal microbiota (Carding et al. 2015). Often underlying gastrointestinal dysfunction are changes in the intestinal microbiome (dysbiosis), especially disturbances in the quantitative and qualitative composition of microbes colonizing the gastrointestinal mucosa (Sekirov et al. 2010; Gomaa 2020). These microbes, owing to their interactions and action on the host, can take part in numerous physiological processes, including digestion, stimulation of local and systemic defence mechanisms, maintenance of homeostasis, and other phenomena determining the normal development of the body (Belkaid and Hand 2014; Rooks and Garrett 2016). Disturbances of the intestinal microbiome have long been known to accompany many disease states, including diabetes and obesity, as well as diseases with inflammation (Musso et al. 2010). Recently published results have shown that the intestinal microbiota also plays an important role in the development and functioning of the central nervous system (CNS) and can influence cognitive functions by acting on metabolic, neuroendocrine, and immune pathways (Carabotti et al. 2015; Zhu et al. 2017; Cerdó et al. 2020). These studies are evidence of two-way communication between the brain and the intestinal microbiota, known as the microbiota–gut–brain axis (Carabotti et al. 2015; Appleton 2018; Chakrabarti et al. 2022). The effects of the intestinal microbiome on the nervous system are usually multifaceted and include its effect on the sensory nerve fibres, including the vagus nerve, which mediate transmission of information to the CNS and decrease the perception of visceral pain (Wang and Kasper 2014; Moloney et al. 2016; Mitrea et al. 2022). Interestingly, disturbances in the intestinal microbiome can also be associated with mood disorders, depression, and anxiety, which has been demonstrated in human subjects with irritable bowel syndrome (Cryan and O’Mahony 2011; Kumar et al. 2023). One of the strategies currently promoted for restoring the microbiological balance of the intestines is diet supplementation with probiotics containing selected strains of various microbial species (Hemarajata and Versalovic 2013). The beneficial effect of probiotic microbes on the body is manifested as regulation of intestinal function, stabilization and maintenance of the balance between pathogenic and saprophytic microbes and stimulation of enterocyte development. Probiotics take also part in the regulation of gastrointestinal motility, increase digestion and absorption of proteins, carbohydrates and fats, and produce biologically active compounds such as enzymes and vitamins (Wang et al. 2021). Probiotic bacteria also stimulate local (GALT, gut-associated lymphoid tissue) and systemic host immune mechanisms, expressed as modulation of T and B cell functions and stimulation of immunocompetent cells to produce cytokines, which regulate the systemic and local immune response (Hardy et al. 2013; Wang et al. 2021). Another positive effect of probiotics is inhibition of inflammation of the intestinal mucosa through stabilization of the environment of bacteria and proliferation and cytokine activation of NK cells (Cristofori et al. 2021). The multifaceted potential effects of probiotics on the body, especially the hypothesis regarding their interactions with the CNS mediated by the microbiota–gut–brain axis, have prompted the implementation of new methods of prevention and treatment of emotional and mental disorders in people, involving the use of psychobiotics as diet supplements (Dinan 2013; Sarkar et al. 2016; Mörkl et al. 2020; Berding et al. 2023).
The concept of psychobiotics refers to a large group of probiotics containing bacterial strains which have no pro-inflammatory lipopolysaccharide chains and do not induce an acute inflammatory response in the intestines or prebiotics which during fermentation of food in the intestines induce changes in the composition or activity of the bacteria constituting the microbiome. Both groups of microbes, when ingested in appropriate quantities, exert positive psychiatric effects in psychopathology (Bermúdez-Humarán et al. 2019; Del Toro-Barbosa et al. 2020; Oroojzadeh et al. 2022). The mechanism of action of psychobiotics is not fully known but is assumed to rely on stimulation of the intestinal nervous system, the immune and endocrine systems, and metabolic processes. The use of psychobiotics in humans has also been shown to influence psychophysiological markers of depression and anxiety, as well as inflammation (Sarkar et al. 2016; Gualtieri et al. 2020). Underlying this effect is the influence of the hypothalamic–pituitary–adrenal axis (HPA) on the stress response, a reduction in systemic inflammation, direct effects on the immune system, and also synthesis of neurotransmitters, proteins, and short-chain fatty acids (Dinan 2013; Sarkar et al. 2016; Cheng et al. 2019; Del Toro-Barbosa et al. 2020; Zielińska et al. 2022).
The bacterial strains most often used to produce psychobiotics include Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1 (Allen et al. 2016; Kelly et al. 2017; Forssten et al. 2022). Bravo et al. (2011) demonstrated that the use of Lactobacillus rhamnosus JB-1 in mice reduced the level of corticosterone in the blood and mitigated depressive and anxious behaviours by reducing expression of the GABAB1β receptor in the hippocampus and amygdala. Diet supplementation with Lactobacillus rhamnosus JB-1 also affected metabolic processes in the brain of mice with stress-associated anxiety disorders, resulting in a reduction in stress severity measured using behavioural tests (Xu et al. 2022). In addition, diet supplementation with this probiotic decreased the response of the HPA axis to stress and expression of specific GABA receptors in individual regions of the brain (Kochalska et al. 2020; Chudzik et al. 2022). Bharwani et al. (2017) and Marin et al. (2017) used a model of chronic psychosocial stress to show that L. rhamnosus JB-1 alleviates anxiety-like behaviours, reduces deficiencies in social interactions, and exerts an immunoregulatory effect. Recent research indicates that the development of psychological disorders such as depression is linked to an increase in inflammation and activation of pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-1β, and tumour necrosis factor α (TNF-α), which indirectly affect the composition of the intestinal microbiome (Lotrich 2015; Maeng and Hong 2019). The use of Lactobacillus rhamnosus JB-1 in mice, on the other hand, reduced the concentration of pro-inflammatory cytokines and mitigated anxiety disorders (Mindus et al. 2021; Chudzik et al. 2022).
Similar effects have been shown for Bifidobacterium longum Rosell®-175. The use of this strain in mice in combination with Lactobacillus helveticus R0052 influenced the HPA axis in a state of chronic stress in mice, alleviating its effects (Ait-Belgnaoui et al. 2014). The combined use of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 in rats and humans reduced cortisol concentrations in the urine, which confirms the anti-anxiety and anti-stress effects of these microbes (Messaoudi et al. 2011).
The multifaceted effects of psychobiotics containing Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1 are linked to stimulation of organ metabolism and the immune and endocrine systems, and also result from activation of specific genes located in the brain, endocrine glands or other tissues (Poluektova et al. 2021). The mechanisms causing metabolic changes in the brain and resulting in a change in the protein profile as a consequence of diet supplementation with psychobiotics containing Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 are as yet unknown. We hypothesized that supplementation of the diet of mice with Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 modifies the expression of brain proteins involved in metabolic and immune processes. The aim of the study was to identify proteins synthesized in the brain of mice fed a diet supplemented with Lactobacillus rhamnosus JB-1 or Bifidobacterium longum Rosell®-175, to determine the differences in the expression of these proteins in comparison with the control group. The results should provide the first scientific evidence of differentiation of the proteomic profile of the brain as a result of diet supplementation with psychobiotics.
Materials and methods
Animals
Thirty male albino Swiss mice were used in this study. The animals were purchased from a licensed breeder (Kołacz, Laboratory Animals Breeding, Warsaw, Poland) at age of 5–6 weeks and were kept under controlled environmental conditions (21–24 °C; 45–65% humidity; 12-h light/dark cycle; light on at 6:00 a.m.) with free access to tap water and standard laboratory chow (Agropol S.J., Motycz, Poland). They were housed in groups in standard transparent cages (37 cm × 21 cm × 14 cm) and habituated for 7 days before starting the treatment. Housing and experimental procedures were performed in accordance with the EU council directive 2010/63/EU and Polish legislation concerning animal experimentation. The experimental protocol was approved by the Local Ethical Committee in Lublin, Poland (license no 65/2022).
Bacterial preparation
Lactobacillus rhamnosus JB-1 (LR-JB1™) was gifted from Prof. Greg J. Stanisz. Bifidobacterium longum Rosell®-175 was obtained courtesy of SANPROBI Sp. z o.o., Sp. k. (Szczecin, Poland). Bacterial strains were stored as a frozen stock at − 80 °C in Man-Rogosa-Sharpe liquid medium (MRS broth; Difco Laboratories, Detroit, USA) containing 20% glycerol. From frozen stocks, bacteria were sub-cultured (overnight, in anaerobic conditions, 37 °C) in the MRS medium supplemented with 0.05% l-cysteine-HCl. The overnight cultures were transferred to fresh MRS broth (4.5 L) and again incubated at 37 °C for 48 h in anaerobic conditions (under mineral oil). After 2 days, cells were harvested from the growth medium by centrifugation at 9000 rpm for 20 min. The pellets were washed three times with sterile PBS buffer and re-suspended in sterile PBS (450 mL). The turbidity of bacterial suspension was compared to the McFarlands scale and its dilution was made in sterile PBS buffer to 1 × 1010 cfu/mL (2 × 109 cfu/0.2 mL). The final bacterial suspensions were bottled into 50-mL Falcon tubes and stored at − 20 °C until the mice were fed.
Treatment
Mice were divided into three groups (n = 10) and administered with: (Group I) 200 µL of PBS (control group), (Group II) 2 × 109 cfu of Bifidobacterium longum Rosell®-175 in 200 µl PBS, and (Group III) 2 × 109 cfu of Lactobacillus rhamnosus JB-1 in 200 µl PBS of by oral gavage every 24 h for a total of 28 days. See Fig. 9.
Tissue collection
Two hours after the last treatment, the animals were sacrificed and the brains were rapidly dissected out and washed out in ice-sold saline (87 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 0.5 mM CaCl2, 7 mM MgSO4, 25 mM glucose, 75 mM sucrose, pH 7.4). Next the brain regions, hippocampus, from each animal were isolated and stored at − 80 °C until proteomic analysis according to the methods described by Wiśniewski and Gaugaz (2015) and Haas-Neill et al. (2022).
Protein extraction
Hippocampus was cut into small pieces, washed in 0.9% NaCl, and homogenized (T10 basic IKA, Germany) in TRIS–HCl (1.5 M in water, pH 8.8). The samples were subsequently purified, desalted, and concentrated using Amicon Ultra-0.5 3 kDa centrifugal filter units (Merck KGaA, Darmstadt, Germany). Next, 180 µg of protein pellets were obtained using a precipitation kit (Ready-Prep™ 2-D Cleanup Kit, Bio-Rad, Warsaw, Poland) and dissolved in rehydration buffer (Bio-Rad, Warsaw, Poland). Protein mixtures were dropped onto a rehydration plate and covered with 17-cm immobilized pH gradient (IPG) linear strips for isoelectric focusing (ReadyStrip IPG, pH 3–10, Bio-Rad, Warsaw, Poland). The strips were overlaid with mineral oil to prevent them from drying out (Mineral oil, Bio-Rad, Warsaw, Poland). The strips were left to rehydrate for 12 h. Next, strips with soaked proteins were put in an IEF-100 Hoefer apparatus (Hoefer IEF100, Hoefer, Inc., Holliston, MA, USA) for electrophoretic isofocusing under the following conditions: 250 V/30 min; 10,000 V/3 h; 60 kV/h, with a current limit of 50 µA/strip. Before the second dimension, strips with focused proteins were equilibrated in 1,4-dithiothreitol and iodoacetamide solutions. Then, the strips were transferred onto 12.5% polyacrylamide gels and subjected to the second dimension of electrophoresis under 600 V/30 mA/100 W in an electrophoretic chamber (PROTEAN® II xi, Bio-Rad, Warsaw, Poland). After separation was completed, the gels were subjected to a standard silver-staining process in the presence of formaldehyde. Next, the gels were digitalized by scanning (Image Scanner III, GE Healthcare, Warsaw, Poland) and processed with Delta2D software (version 4.7, DECODON, Greifswald, Germany). Gel images were warped, which means that spots of the same protein had the same position across all gels in the experiment, and were fused. A fused image can be defined as a protein map containing every protein spot obtained during the experiment. Expression ratios were generated after the assumptions were checked based on the Shapiro–Wilk test (α = 0.05) and statistics were calculated for normalized volumes by one-way ANOVA (P value ≤ 0.05) and a post hoc Tukey comparison test (P value ≤ 0.05).
Protein identification
Selected proteins which were statistically significant were cut from the gels, destained, reduced, and alkylated using dithiothreitol and iodoacetamide solutions. Next, the gel fragments were digested with trypsin solution in 50 mM bicarbonate buffer at 37 °C for 12 h (Promega, Trypsin Gold, Mass Spectrometry Grade, Technical Bulletin). The resulting peptides were eluted from the gel pieces with a water/acetonitrile/TFA solution (v:v 450:500:50) by triple extraction. Peptide mixtures were purified and concentrated using C18 Zip-TIP pipette tips according to the manufacturer’s guidelines (Merck Chemicals, Billerica, MA, USA, PR 02358, Technical Note). Next, the peptide solutions and standard solution (Peptide Calibration Standard II, Bruker, Bremen, Germany) were spotted on an Anchor Chip MALDI plate (Bruker, Bremen, Germany) and covered with 1 µL of α-cyano-4-hydroxycinnamic acid matrix (HCCA, Bruker, Bremen, Germany). Mass spectra were obtained in positive reflector mode within the 700–4000 m/z range using an Ultraflextreme MALDI TOF/TOF spectrometer (Bruker, Bremen, Germany) and flexControl 3.3 software (Bruker, Bremen, Germany). The resulting spectra were smoothed and baseline corrected. The peak list generated in flexAnalysis 3.0 software (Bruker, Bremen, Germany) for a signal-to-noise ratio of > 3 was transferred to BioTools 3.2 (Bruker, Bremen, Germany) and compared to the Swiss-Prot database (www.uniprot.org) in Mascot 2.2 software (Matrix Science, Boston, MA, USA), restricted to ‘mus musculus’, with maximum error of 0.3 Da and carbamidomethylation of cysteine as an obligatory modification. Results with a Mascot score above 55 were considered statistically significant (P ≤ 0.05); otherwise, the combined ion spectra of selected peptides were obtained using the LIFT mode and subjected to MALDI TOF/TOF identification.
Statistical analysis
On the basis of spot volumes, differences in protein expression between the test groups were analysed by one-way analysis of variance ANOVA with alpha critical value P ≤ 0.05 based on F-distribution in Delta2D 4.7.0 (DECODON, Greifswald, Germany) and a post hoc Tukey comparison test. A spot intensity ratio higher than 1.3 (upregulated) or lower than 0.67 (downregulated) was the basis for protein identification. The results were presented in Figs. 1, 2, 3, 4, 5, 6, 7, and 8 and Table 1.
Fused image showing condensed spot patterns from the experiment. The differentially expressed proteins are marked with circles. Proteins were separated in the first dimension by isoelectric focusing over the isoelectric point (pI) range 3–10. The second dimension was performed using a 12.5% sodium dodecyl sulphate polyacrylamide gel. Gels were silver stained, digitized, and processed in Delta2D software (version 4.7 DECODON Greifswald, Germany)
Mean volumes (%) of protein spots in the experimental and control groups. Statistically different proteins are compared, and ratio parameters (Rt) are given. TK, transketolase; CAT, catalase. Significant differences assessed using the ANOVA test and Tukey’s post hoc test are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01
Mean volumes (%) of protein spots in the experimental and control groups. Statistically different proteins are compared, and ratio parameters (Rt) are given. STXB1, syntaxin-binding protein 1; EIF4H, eukaryotic translation initiation factor 4H; CALCY, neuron-specific vesicular protein calcyon; SFRP3, secreted frizzled-related protein 3. Significant differences assessed using the ANOVA test and Tukey’s post hoc test are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01
Mean volumes (%) of protein spots in the experimental and control groups. Statistically different proteins are compared, and ratio parameters (Rt) are given. OBP2, odorant-binding protein 2; CAII, carbonic anhydrase 2. Significant differences assessed using the ANOVA test and Tukey’s post hoc test are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01
Mean volumes (%) of protein spots in the experimental and control groups. Statistically different proteins are compared, and ratio parameters (Rt) are given. IL-18, interleukin-18; CLEC1A, C-type lectin domain family 1 member A. Significant differences assessed using the ANOVA test and Tukey’s post hoc test are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01
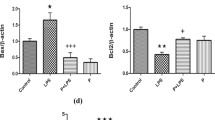
Mean volumes (%) of protein spots in the experimental and control groups. Statistically different proteins are compared, and ratio parameters (Rt) are given. SPRN, shadow of prion protein; NPM2, nucleoplasmin-2. Significant differences assessed using the ANOVA test and Tukey’s post hoc test are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01
Mean volumes (%) of protein spots in the experimental and control groups. Statistically different proteins are compared, and ratio parameters (Rt) are given. GLRA4, glycine receptor α4 subunit. Significant differences assessed using the ANOVA test and Tukey’s post hoc test are marked with asterisks: *P ≤ 0.05; **P ≤ 0.01
Results
Protein identification
Among the proteins in the samples analysed, we focused only on those with differential electrophoretic spots. MALDI-TOF mass spectrometry identified 13 statistically significant proteins (Table 2). Stains were positively identified as the enzyme transketolase (TK); odorant-binding protein 2 (OBP2), a small extracellular protein of the lipocalin superfamily; the regulator protein syntaxin-binding protein 1 (STXB1); the enzyme catalase (CAT); the transport protein glycine receptor α4 subunit (GlRA4); secreted frizzled-related protein 3 (SFRP3); the enzyme carbonic anhydrase 2 (CAII); the thermostable acidic protein nucleoplasmin-2 (NPM2); eukaryotic translation initiation factor 4H (EIF4H); shadow of prion protein (SPRN); C-type lectin domain family 1 member A (CLEC1A); interleukin-18 (IL-18); and neuron-specific vesicular protein calcyon (CALCY). Table 1 lists protein names, UniProt base accession numbers, ANOVA P values and Tukey’s HSD P value. See also Figs. 1 and 2 and Table 2.
Protein profile of the hippocampus
The results indicate statistically significantly higher (P ≤ 0.05) concentrations of TK in the hippocampus of mice in the control group compared to both experimental groups. The mean volume of CAT was statistically significantly higher (P ≤ 0.05) in the hippocampus of mice receiving a Lactobacillus rhamnosus JB-1 suspension compared to the control group. No statistically significant differences were observed in the mean volume of catalase in the group of mice receiving the Bifidobacterium longum Rosell®-175 suspension compared to the control group (Fig. 3).
Statistically significantly lower (P ≤ 0.05) mean volumes of STXB1 and EIF4H were observed in the hippocampus of mice in both experimental groups compared to the control group. The mean volumes of CALCY and SFRP3 in the hippocampus of mice from both experimental groups were statistically significantly higher (P ≤ 0.05) than in the control group. However, there were no statistically significant differences in the mean volumes of these proteins between the two experimental groups (Fig. 4).
In the case of CALCY, it was not possible to obtain statistically significant identification results with the MALDI TOF method. A score of 54 was achieved (a score above 55 is necessary for statistically significant identification). Nevertheless, three peptide matches were confirmed during tandem mass spectrometry in triplicate. Statistically significant identification may have been prevented by the considerable post-translational modifications resulting from psychobiotics administration.
The mean volume of OBP2 in the group of mice receiving the Lactobacillus rhamnosus JB-1 suspension was statistically significantly lower (P ≤ 0.05) than in the control group. However, the mean volume of CAII was statistically significantly lower (P ≤ 0.05) in the tissues of mice receiving the Bifidobacterium longum Rosell®-175 suspension compared to the group of mice receiving the Lactobacillus rhamnosus JB-1 suspension (Fig. 5).
No statistically significant differences in the mean volume of IL-18 were observed between the control and experimental groups. However, the mean volume of this protein was statistically significantly higher (P ≤ 0.05) in the group of mice receiving the Bifidobacterium longum Rosell®-175 suspension compared to the group supplemented with the Lactobacillus rhamnosus JB-1 suspension. The mean volume of CLEC1A was statistically significantly higher (P ≤ 0.05) in the control group compared to the group supplemented with Bifidobacterium longum. However, no statistically significant differences in the mean volume of this protein were observed between the experimental groups or between the Lactobacillus rhamnosus JB-1-supplemented group and the control group (Fig. 6).
Compared to the control group, statistically significantly lower (P ≤ 0.05) mean volume of shadow of prion protein (SPRN) was observed in the hippocampus of mice from the Lactobacillus rhamnosus JB-1 supplemented group. Similarly, significantly lower mean volume of NPM2 was observed in both experimental groups compared to the control group. No statistically significant differences in mean volumes of NPM2 were observed between the experimental groups (Fig. 7).
Statistically significant differences (P ≤ 0.05) in the mean volume of GLRA4 were observed in the hippocampus of mice in both experimental groups compared to the mean volume of this protein in the tissues of mice from the control group. The obtained results indicate that the average volume of GLRA4 in the hippocampus of mice from the group supplemented with Lactobacillus rhamnosus JB-1 was statistically significantly lower (P ≤ 0.05) compared to the average volume of these proteins in mice from the control group. However, in the group of mice supplemented with Bifidobacterium longum Rosell®-175, a statistically significantly higher (P ≤ 0.05) mean volume of GLRA4 was observed compared to the control group (Fig. 8).
Discussion
The study provided the first evidence of differentiation of the proteomic profile of the hippocampus of mice in response to diet supplementation with Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175. The proteins which differentiate mice from the experimental groups and the control perform numerous functions in the body, taking part in important cellular processes (Fig. 9, Tables 1 and 2).
Changes were shown in the expression of SFRP3, a secreted frizzled-related protein. SFRPs are a family of soluble proteins with the ability to inhibit signalling pathways by binding to Wnt ligands and/or Fz receptors (serpentine receptors, called frizzleds) (Bovolenta et al. 2008). It should be noted that in various experimental models SFRP3 acts as an antagonist or agonist of the Wnt signalling pathway, causing an increase in the concentration of this protein in states of tumour progression and metastasis (Pećina-Šlaus et al. 2016). Wnt signalling regulates brain development processes during the embryonic period and controls proliferation and differentiation of progenitor cells in the nervous system in the postnatal period (Patapoutian and Reichardt 2000; Shu et al. 2018). Neurogenesis in the hippocampus proceeds in parallel with peripheral neurogenesis and is regulated by physiological and pathological stimuli, which influence neuron activity (Shohayeb et al. 2018; Fares et al. 2019). Stimuli accelerating neuronal maturation and integration include physical activity, learning processes, and the occurrence of pathological seizures, as well as the psychobiotics L. rhamnosus JB-1 and B. longum Rosell®-175 used in the study, which stimulate synthesis of neuroactive proteins through the microbiota–gut–brain axis. The increase in the expression of these proteins in both experimental groups may suggest that they could be involved in the activation and maturation of nerve cells. Previous research indicates that bacteria such as L. rhamnosus strain GG modulate Wnt/β-catenin signalling pathways in various cell lines, including cancer cells, increasing expression of SFRP (Aherian-Esfahani et al. 2016). The increase in the expression of SFRP3 in the present study following administration of B. longum and L. rhamnosus may also be related to the regulation of the expression of genes of Wnt-associated signalling pathways. Wnt signalling plays an important role in regulating early brain development, cell migration, dendrite morphogenesis, and synapse formation, as well as in controlling cognitive functions, thus preventing neurodevelopmental, neurological, and neurodegenerative disorders (Hussaini et al. 2014; Seib et al. 2013). The latest data suggest that SFRP3 is an endogenous antagonist of Wnt, and a decrease in its secretion stimulates neurogenesis in the hippocampus (Jang et al. 2013a, b) and promotes antidepressant activity in mice and humans (Jang et al. 2013a). Nevertheless, the concentration of SFRP3 mRNA in the mouse hippocampus remains unchanged for its entire life (Jang et al. 2013a, b; Cho et al. 2019) and exerts a neuroprotective effect by promoting myelination. The results of our experiment indicate the need for continued research to assess the effect of SFRP3 on processes of cellular signalling, myelination, and neurogenesis, which is crucial to explaining the mechanisms and processes associated with neurodevelopmental disorders and to the development of therapeutic strategies to prevent a decline in cognitive functions.
In our experiment, the psychobiotics Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 also significantly affected CALCY. Previous research indicates that CALCY plays an important role in brain function and in the development of psychological disorders by influencing neuron development and synaptic plasticity (Li et al. 2011; Chander et al. 2019). Specifically, it is involved in endocytosis within the synapses, mediated by clathrin, which is essential to synaptic transmission and optimization of the range of released neurotransmitters, as well as in dopamine-related signalling and dopamine activity (Xiao et al. 2006). Neurotransmission processes associated with dopamine affect various brain functions, such as motor control and cognitive processes (Seamans and Yang 2004). Intestinal bacteria, including the psychobiotics used in the experiment, have been shown to produce numerous neurotransmitters in metabolic processes, including dopamine, noradrenaline, serotonin, GABA, and acetylcholine (Lyte 2011). Liu et al. (2016) demonstrated that administration of Lactobacillus plantarum PS128 to mice increases dopamine and serotonin concentrations in the prefrontal cortex and striatum. Therefore, it is possible that the psychobiotics used in the experiment, by producing neurotransmitters and stimulating expression of CALCY, influence synaptic transmission and coordinate signal processing and mechanisms of intercellular communication in the central and peripheral nervous system. A precise understanding of the mechanisms underlying these phenomena requires in-depth immunohistochemical and metabolic analysis of the functioning of the mouse CNS.
The study showed that administration of Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 to mice as diet supplements also alters expression of isoform II of carbonic anhydrase (CAII). Carbonic anhydrases (CA) are a large group of zinc metalloenzymes present in mammals in 14 different isoforms and catalysing reversible hydration of carbon dioxide to bicarbonate (Boone et al. 2013). They perform various functions in the body, including regulation of the water and electrolyte balance and pH homeostasis and a role in numerous metabolic pathways, such as gluconeogenesis, lipogenesis, and ureagenesis. In addition, they take part in bone resorption and calcification and in the formation of cerebrospinal fluid. One of the cytosolic isoenzymes of CA is the isoform CA II, expressed in the CNS (Lemon et al. 2021). CA II is present in the myelin and glial cells, microglia, choroidal epithelium, astrocytes and neurons, and in mice primarily in the oligodendrocytes and myelin sheaths (Lakkis et al. 1997). CA activity is involved in the regulation of extracellular pH in the brain, which affects neuronal activity. The increase in the expression of this protein in mice receiving B. longum Rosell®-175 may indicate effective modulation of the pH of the extracellular fluid, which contributes to generation of optimal synaptic currents and strengthens and maintains the metabolic activity of neurons. It is worth noting that administration of compounds such as amino acids to mice increases CA activity in the brain, thereby improving memory. Our results suggest that the increased expression of CAII in the hippocampus following administration of B. longum Rosell®-175 to mice stimulates activation of the brain through memory formation, processing and enhancement. In addition, Giacobini (1987) suggest that high CA concentrations are favourable in the early stages of neuron growth and maturation, and thus the high expression of this protein in the group of mice receiving B. longum Rosell®-175 may be linked to neuronal development and the acquisition of functions by CNS cells. Precise knowledge of the mechanisms underlying these phenomena requires in-depth research on the effects of psychobiotics on the functioning of the CNS of mice, including the hippocampus.
Analysis of the results of the study indicated decreased expression of OBP2 following the use of Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 in the diet of mice. OBP in vertebrates is expressed in the nasal epithelium, where it mediates olfactory transduction, binding and transporting hydrophobic and volatile aromatic molecules and pheromones through watery mucus (Pelosi et al. 2005; Forêt and Maleszka 2006). Binding of aromatic substances involves OBP2, a soluble carrier protein which shows the strongest affinity for long-chain aldehydes and fatty acids (Tcatchoff et al. 2006). The mRNA encoding this protein is more strongly expressed in the tissues of the brain, heart, kidney, liver, genitals, and lungs (Yanai et al. 2005). OBP has also been shown to be expressed in the brain tissue of developing insects (Forêt and Maleszka 2006), transporting ligands of various molecules for neuron development and signal transmission, thereby influencing insect behaviour, including adaptation to the environment (Forêt and Maleszka 2006; Guo et al. 2018). OBP has also been shown to exert broad-spectrum antimicrobial effects (Bianchi et al. 2019). The lower expression of this protein in the hippocampus of mice from the experimental groups does not rule out an analogous effect in the case of the microbes used in our study. This phenomenon may be explained by different metabolic pathways in which these microbes are involved, whereby metabolic products other than fatty acids and aldehydes which strongly stimulate OBP are supplied to the CNS.
All biological processes, including metabolic transformations, entail the generation of reactive oxygen species (ROS) and free radicals, which are responsible for the development of oxidative stress (Lushchak 2014). ROS are essential for the functioning of the body, playing the role of signal transmitters, regulating repair processes in cells and gene expression, and taking part in metabolism and redox reactions (Dröge 2002). Excessive ROS cause oxidative stress, leading to damage to cell components and disturbing cellular integrity (Schieber and Chandel 2014). Oxidative stress and ROS play an important role in neurological disorders and age-related cognitive performance, as shown in studies in humans (Dröge 2002; Mariani et al. 2005; Singh et al. 2019). These compounds also modulate synaptic transmission processes (Knapp and Klann 2002; Serrano and Klann 2004) and take part in signalling pathways (D'Autréaux and Toledano 2007), and by acting on the amygdala and hippocampus, they influence behavioural and cognitive functions. Protection against these effects is ensured by the antioxidant system in various tissues and systems, an important component of which is the enzyme CAT. CAT neutralizes O2- anion radicals, hydroxyl radicals and radicals of unsaturated fatty acids and breaks down hydrogen peroxide formed during cellular respiration to molecular oxygen and water, ensuring a state of dynamic balance between the formation and elimination of ROS (Weydert and Cullen 2010). A high CAT concentration has been noted in the liver, kidneys, and erythrocytes, but its presence has been confirmed in the brain as well (Scaglione et al. 2016). Studies on an animal model have shown that the interaction between a probiotic administered with feed and the intestinal microbiome modulates the body’s immune response to various harmful factors, including inflammation and oxidative stress (Zhang et al. 2019). Our study showed a high concentration of this protein in the hippocampus of mice receiving a diet supplemented with the psychobiotics Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175, which confirms that they can stimulate the gut–brain axis and provides evidence of stimulation of the antioxidant system. The efficient antioxidant system, including catalase activity, guarantees neuromodulatory function and maintenance of homeostasis and presumably affects signalling pathways and cognitive functions. This hypothesis is supported by Clausen et al. (2010, 2012) and Olsen et al. (2013), who showed that the use of antioxidants such as CAT mimetics in mice corrects or mitigates cognitive deficits and fear-conditioning deficits caused by oxidative stress and stimulates synaptic plasticity by taking part in neurotransmission. Wang et al. (2009) and Cui et al. (2012) showed that decreased CAT activity is associated with impairment of hippocampus-dependent spatial memory. The results of our study confirm the stimulatory role of psychobiotics in processes limiting stress responses by stimulating production of antioxidant enzymes. It is worth emphasizing that Lactobacillus strains with strong antioxidant properties occur in nature (Kono and Fridovich 1983), and these can be used as active components of probiotic supplements. One of these microbes is L. plantarum, which produces manganese pseudocatalase, an enzyme stimulating conversion of H2O2 to water and oxygen. The biological activity of this enzyme has been shown to be similar to that of haem CAT, which is present in cells (Kono and Fridovich 1983). While these properties have not been assessed for the strains Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 used in our study, this type of effect cannot be ruled out.
Analysis of the results of the study indicates that administration of Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 to mice leads to a reduction in the expression of proteins STXB1, EIF4H, CLEC1A, TK, SPRN, and NPM2.
Clec1A is one of the CLR receptors taking part in the host’s defence against pathogens and is also involved in regulating immune system function and the development of autoimmune and neoplastic processes (Makusheva et al. 2022). This protein also plays an important role in the development of CNS inflammation by upregulating transport of Th17 cells through the blood–brain barrier (Arima et al. 2012; Makusheva et al. 2022). The low expression of this protein in the experimental groups receiving psychobiotics may indicate that there were no ongoing inflammatory processes in the CNS, autoimmune processes, or tissue degradation. In this context, it could be interesting to note the results obtained for the expression of interleukin 18, which was higher in the experimental group receiving Lactobacillus rhamnosus JB-1 than in the control group, but lower in the group receiving Bifidobacterium longum Rosell®-175 than in the controls. IL-18 mediates mechanisms of the innate and acquired immune response and regulates the cellular and humoral immune response (Alboni et al. 2010). In the publish study’s demonstrated that, IL-18 was expressed in all structures of the brain, with the highest concentrations noted in the hypothalamus, hippocampus, and amygdala. Studies of the hippocampus in mice and rats have shown that IL-18 modulates neuronal functions, takes part in synaptic transmission, mediates communication between the central and peripheral nervous system, and regulates the activity of the hypothalamic–pituitary axis (Alboni et al. 2010; Kuwahara-Otani et al. 2017). The increased expression of IL-18 in conjunction with the results obtained for previously described proteins, e.g. CALCY, may suggest that administration of the psychobiotic Lactobacillus rhamnosus JB-1 to mice leads to stimulation of synaptic transmission, which influences neuromodulatory processes, memory, and cognitive functions. An increase in IL-18 expression may also be linked to inflammatory processes and brain damage (Alboni et al. 2010). It is worth emphasizing, however, that the pleiotropic role of IL-18 in CNS development is not fully understood. It is known to inhibit neuron differentiation, inducing the death of nerve cells, and to take part in the formation of neural stem cells in response to trauma (Johansson et al. 1999; Lingenfelter et al. 2011). Lactobacillus strains are also known to have the ability to activate MDC in order to induce an immune response in T cells and to induce the formation and secretion of Th1 cytokines, including IL-18, IFN I and II, and IL-12 (Mohamadzadeh et al. 2005). That study supports the results of our experiment, in which expression of IL-18 was increased in the group of mice receiving Lactobacillus.
We obtained similar results for the expression of the protein GLRA4, a glycine receptor containing subunit α4. Expression of glycine receptors (GlyR) has been shown in the developing brain and in the fully developed spinal cord, hindbrain, cerebellum, and retina. They play an important role in mediation of inhibitory neurotransmission in the brain and spinal cord, and also take part in stimulatory neurotransmission in embryonic neurons (Matzenbach et al. 1994; Harvey et al. 2000; Baer et al. 2009; Lynch 2009). Among the five GlyR subtypes, the function of GLRA4 in people and animals remains unclear, because the human GLRA4 gene is considered a pseudogene (Simon et al. 2004), present in the genome in the form of a neutral sequence with no biological function (Simon et al. 2004; Bar-Shira et al. 2015). Nevertheless, many studies indicate that pseudogenes can perform biological functions and play a role in health and disease (Tam et al. 2008; Pink et al. 2011). Given that most neurons of the CNS are inhibited by glycine (Nicoll et al. 1990), an increase in GLRA4 expression may be linked to inhibition of neurotransmission in the brain of mice. Expression of GLRA4 in the study may not fully reflect its physiological role in the hippocampus of mice following administration of the diet supplement. Increased expression of GLRA4 may be linked to brain development dependent on amino acids released from glial cells, e.g. taurine or β-alanine (Flint et al. 1998; Mori et al. 2002). GLRA4 is found in large quantities in the synapses, where it takes part in regulation of synaptic stimulation and inhibition (Legendre 2001). The synapses in the spinal cord (Jonas et al. 1998), brainstem (Russier et al. 2002), and cerebellum (Dumoulin et al. 2001) include mixed GABA/glycine synapses which can mediate neurotransmission, while activation of GlyR may inhibit GABAARs via a phosphorylation-dependent mechanism (Li et al. 2003). Results reported by Bravo et al. (2011) suggest that Lactobacillus rhamnosus JB-1 exerts a direct effect on the GABAergic system and neurotransmission processes in mice, reducing behaviours associated with depression and anxiety. Similarly, Yunes et al. (2020) found that Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 produce large amounts of GABA, which modulates GABAergic signalling through GABA receptors located on intestinal neurons (Yunes et al. 2016). This demonstrated the antidepressant effect of these receptors in mice, expressed as a reduction in behaviours reminiscent of depression. The increase in GLRA4 expression in our study in the group of mice receiving Lactobacillus may be associated with neural transmission, which ensures the normal function of complex cerebral processes such as neuron excitability, synaptic plasticity, and cognitive functions, e.g. learning and memory. It should be noted that lactic acid bacteria (LAB) of the genus Lactobacillus produce large amounts of GABA during fermentation, and GABA receptors are present in the intestinal microbiota (Yunes et al. 2016). These data in combination with the results of the present study indicate an interdependence between glycine and GABA receptors, which translates to modulation of the ‘stimulation–inhibition’ balance and demonstrates the value of using psychobiotics to treat abnormal behaviour associated with anxiety and depression (Sarkar et al. 2016).
The present study also showed reduced expression of the synaptic protein STXB1, which is essential in neurotransmission processes (Chen et al. 2020). Interacting with and binding syntaxin, this protein takes part in fusion of synaptic vesicles with presynaptic membranes, releasing neurotransmitters (Shen et al. 2015). The lack of the gene encoding Stxbp1 or its low expression even results in complete loss of the release of neurotransmitters and synaptic conduction, and in consequence in brain dysfunctions (Zoghbi and Bear 2012), and various pathological states (Saitsu et al. 2008). Miyamoto et al. (2017) showed that transgenic mice with overexpression of Stxbp1 displayed increased aggression, while mice with a Stxbp1 + / − deficit exhibited impaired neurotransmission-dependent cognitive processes. These studies show that synaptic transmission deficits induced by a deficiency or lack of Stxbp1 can cause disturbances in nervous system development and induce disease and behavioural disorders. In our study, the STXB1 concentration in mice following administration of psychobiotics was statistically significantly lower than in the control group.
Low expression of EIF4H was also shown in the groups of mice receiving psychobiotics. A deficiency of this protein in mice induces growth disorders, changes in the brain in the form of neuronal morphology disorders, and behavioural disorders associated with learning and memory, indicating damage to the hippocampus and amygdala (Capossela et al. 2012; Kats and Klann 2019). Due to the lack of literature data on the function of EIF4H in health and disease, it is not possible to state whether a lack or decreased amount of EIF4H causes abnormalities in the CNS, expressed as developmental and functional disorders. EIF4H is present in all structures of the brain, including in the synapses, and is involved in protein synthesis and mRNA translation. Reduced expression of this protein affects synaptic protein synthesis, which may underlie the onset of neuronal signalling disorders and lead to changes in animal behaviour (Capossela et al. 2012). Further research on the role of EIF4H in neuronal development and CNS functioning is needed.
Proteins for which we showed no differences in expression between the experimental groups and the control also include NPM2 and SPRN. The physiological function of SPRN in the CNS is not fully known. SPRN is a protein with properties similar to those of PrPc, so it is presumed that it may also take part in the development of neurodegenerative diseases in humans, such as Alzheimer’s disease (Passet et al. 2020). In mice, this protein is present in two regions of the brain—in the Purkinje cells in the cerebellum and in the pyramidal cells of the hippocampus, and an increase in its expression is linked to numerous functions performed during embryonic development and to tissue growth and development (Lloyd et al. 2009). NPM2 is involved in chromatin condensation, and a deficiency of this protein causes developmental defects and embryo mortality (Burns et al. 2003). However, the exact function of this protein in the CNS of mice has not yet been described. Low expression of both proteins demonstrates that they are mainly responsible for cell development during the embryonic period, and their concentrations are higher in reproductive cells and organs (Lingenfelter et al. 2011). An increase in the concentrations of these proteins may be associated with the development of diseases impairing neuron function and morphology, which may be linked to pathological cell proliferation.
Vitamins take part in many physiological and biochemical processes. One such vitamin is thiamine (vitamin B1), a deficiency of which causes metabolic disorders associated with a lack of production of enzymes such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complex (Whitfield et al. 2018). Adequate thiamine intake is also essential for the CNS, as it performs neuromodulatory functions in the acetylcholine neurotransmitter system and takes part in the structure and functions of cell membranes, including of neurons and neuroglia (Mkrtchyan et al. 2015). Converted to thiamine pyrophosphate, thiamine also acts as a coenzyme for TK. TK is the key enzyme in the pentose phosphate pathway (PPP), which synthesizes ribose-5-phosphate and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) (Zhao et al. 2014). Thiamine deficiency has been shown to reduce transketolase activity, which impairs the energy metabolism of the cell, reduces the viability of brain cells, and contributes to the onset of neurodegenerative diseases (Liu et al. 2017; Håglin et al. 2020) and neurological disorders associated with reduced behavioural and cognitive functions. The experiment showed a very low concentration of this enzyme in the hippocampus of mice receiving Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 compared to controls. Research on the effect of thiamine-producing lactic acid bacteria (LAB) on the intestinal microbiota, the microbiota–gut–brain axis, and neuron metabolism in the CNS has demonstrated that L. rhamnosus is able to produce thiamine intracellularly (Teran et al. 2021). This suggests that these bacteria could be used as an alternative to pharmaceuticals to support the treatment and prevention of neurodegenerative diseases. However, none of the bacterial strains studied thus far has led to the production of an adequate concentration of thiamine (Masuda et al. 2012), as confirmed by our own results. The low content of TK in the hippocampal cells may indicate increased demand for aerobic metabolism and synthesis of neurotransmitters in developing nerve cells in mice during their growth and development.
Conclusion
The results of the study suggest that the use of the psychobiotics Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 as diet supplements in mice enhances expression of proteins involved in the activation and maturation of nerve cells, as well as myelination and homeostatic regulation of neurogenesis. An increase in the expression of these proteins plays an important role in processes associated with neurodevelopmental, psychoactive, anxiety and depressive disorders and can be exploited in therapeutic strategies to prevent a reduction in cognitive functions. The results also indicate that the psychobiotics tested cause a decrease in the expression of proteins associated with CNS development and in synaptic transmission, thereby reducing the capacity for communication between nerve cells. Practical application of the research will require a better understanding of the mechanisms underlying the effects of psychobiotic microbes and their metabolites on neurons and neuroimmunomodulation processes. The results of the study indicate that psychobiotic bacteria can be useful in the development of biotherapeutics which could be used in auxiliary treatment of neurological disorders.
Data availability
All data generated during the current study are included in this article and are available from the first author.
References
Aherian-Esfahani Z, Abedin-Do A, Nouri Z, Mirfakhraie R, Ghafouri-Fard S, Motevaseli E (2016) Lactobacilli differentially modulate mTOR and Wnt/ β-Catenin pathways in different cancer cell lines. Iran J Cancer Prev 9(3):e5369. https://doi.org/10.17795/ijcp-5369
Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Houdeau E, Theodorou V, Tompkins T (2014) Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil 26:510–520. https://doi.org/10.1111/nmo.12295
Alboni S, Cervia D, Sugama S, Conti B (2010) Interleukin 18 in the CNS. J Neuroinflammation 7:9. https://doi.org/10.1186/1742-2094-7-9
Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, Murphy E, Cryan JF, Dinan TG, Clarke G (2016) Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 6:e939. https://doi.org/10.1038/tp.2016.191
Appleton J (2018) The gut-brain axis: influence of microbiota on mood and mental health. Integr Med (encinitas) 17(4):28–32
Arima Y, Harada M, Kamimura D, Park JH, Kawano F, Yull FE, Kawamoto T, Iwakura Y, Betz UA, Márquez G, Blackwell TS, Ohira Y, Hirano T, Murakami M (2012) Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell 148(3):447–457. https://doi.org/10.1016/j.cell.2012.01.022
Baer K, Waldvogel HJ, Faull RL, Rees MI (2009) Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci 2:25. https://doi.org/10.3389/neuro.02.025.2009
Bar-Shira O, Maor R, Chechik G (2015) Gene expression switching of receptor subunits in human brain development. PLoS Comput Biol 11(12):e1004559. https://doi.org/10.1371/journal.pcbi.1004559
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157(1):121–141. https://doi.org/10.1016/j.cell.2014.03.011
Berding K, Bastiaanssen TFS, Moloney GM, Boscaini S, Strain CR, Anesi A, Long-Smith C, Mattivi F, Stanton C, Clarke G, Dinan TG, Cryan JF (2023) Feed your microbes to deal with stress: a psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol Psychiatry 28:601–610. https://doi.org/10.1038/s41380-022-01817-y
Bermúdez-Humarán LG, Salinas E, Ortiz GG, Ramirez-Jirano LJ, Morales JA, Bitzer-Quintero OK (2019) From probiotics to psychobiotics: live beneficial bacteria which act on the brain-gut axis. Nutrients 11:890. https://doi.org/10.3390/nu11040890
Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P (2017) Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med 15:7. https://doi.org/10.1186/s12916-016-0771-7
Bianchi F, Flisi S, Careri M, Riboni N, Resimini S, Sala A, Conti V, Mattarozzi M, Taddei S, Spadini C, Basini G, Grolli S, Cabassi CS, Ramoni R (2019) Vertebrate odorant binding proteins as antimicrobial humoral components of innate immunity for pathogenic microorganisms. PLoS ONE 14(3):e0213545. https://doi.org/10.1371/journal.pone.0213545
Boone CD, Habibzadegan A, Gill S, McKenna R (2013) Carbonic anhydrases and their biotechnological applications. Biomolecules 3(3):553–562. https://doi.org/10.3390/biom3030553
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci 121:737–746. https://doi.org/10.1242/jcs.026096
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108:16050–16055. https://doi.org/10.1073/pnas.110299910
Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, Katileviciute A, Khoja S, Kodzius R (2020) Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health 8:574111. https://doi.org/10.3389/fpubh.2020.574111
Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, Eppig JJ, Matzuk MM (2003) Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 300(5619):633–636. https://doi.org/10.1126/science.1081813
Capossela S, Muzio L, Bertolo A, Bianchi V, Dati G, Chaabane L, Godi C, Politi LS, Biffo S, D’Adamo P, Mallamaci A, Pannese M (2012) Growth defects and impaired cognitive-behavioral abilities in mice with knockout for Eif4h, a gene located in the mouse homolog of the Williams-Beuren syndrome critical region. Am J Pathol 180(3):1121–1135. https://doi.org/10.1016/j.ajpath.2011.12.008
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203–209
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26:26191. https://doi.org/10.3402/mehd.v26.26191
Cerdó T, Diéguez E, Campoy C (2020) Impact of gut microbiota on neurogenesis and neurological diseases during infancy. Curr Opin Pharmacol 50:33–37. https://doi.org/10.1016/j.coph.2019.11.006
Chakrabarti A, Geurts L, Hoyles L, Iozzo P, Kraneveld AD, La Fata G, Miani M, Patterson E, Pot B, Shortt C, Vauzour D (2022) The microbiota-gut-brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol Life Sci 79(2):80. https://doi.org/10.1007/s00018-021-04060-w
Chander P, Kennedy MJ, Winckler B, Weick JP (2019) Neuron-specific gene 2 (NSG2) encodes an AMPA receptor interacting protein that modulates excitatory neurotransmission. eNeuro 6(1):ENEURO.0292–18.2018. https://doi.org/10.1523/ENEURO.0292-18.2018
Chen W, Cai ZL, Chao ES, Chen H, Longley CM, Hao S, Chao HT, Kim JH, Messier JE, Zoghbi HY, Tang J, Swann JW, Xue M (2020) Stxbp1/Munc18-1 haploinsufficiency impairs inhibition and mediates key neurological features of STXBP1 encephalopathy. eLife 9:e48705. https://doi.org/10.7554/eLife.48705
Cheng LH, Liu YW, Wu CC, Wang S, Tsai YC (2019) Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J Food Drug Anal 27:632–648. https://doi.org/10.1016/j.jfda.2019.01.002
Cho CH, Yoo KH, Oliveros A, Paulson S, Hussaini SMQ, van Deursen JM, Jang MH (2019) sFRP3 inhibition improves age-related cellular changes in BubR1 progeroid mice. Aging Cell 18(2):e12899. https://doi.org/10.1111/acel.12899
Chudzik A, Słowik T, Kochalska K, Pankowska A, Łazorczyk A, Andres-Mach M, Rola R, Stanisz GJ, Orzyłowska A (2022) Continuous ingestion of Lacticaseibacillus rhamnosus JB-1 during chronic stress ensures neurometabolic and behavioural stability in rats. Int J Mol Sci 23(9):5173. https://doi.org/10.3390/ijms23095173
Clausen A, Doctrow S, Baudry M (2010) Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging 31(3):425–433. https://doi.org/10.1016/j.neurobiolaging.2008.05.009
Clausen A, Xu X, Bi X, Baudry M (2012) Effects of the superoxide dismutase/catalase mimetic EUK-207 in a mouse model of Alzheimer’s disease: protection against and interruption of progression of amyloid and tau pathology and cognitive decline. J Alzheimers Dis 30(1):183–208. https://doi.org/10.3233/JAD-2012-111298
Cohen BE, Edmondson D, Kronish IM (2015) State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 28:1295–1302. https://doi.org/10.1093/ajh/hpv047
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386. https://doi.org/10.3389/fimmu.2021.578386
Cryan JF, O’Mahony SM (2011) The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23:187–192. https://doi.org/10.1111/j.1365-2982.2010.01664.x
Cui Y, Shu Y, Zhu Y, Shi Y, Le G (2012) High-fat diets impair spatial learning of mice in the Y-maze paradigm: ameliorative potential of α-lipoic acid. J Med Food 15(8):713–717. https://doi.org/10.1089/jmf.2011.1970
Darwish M, Hattori S, Nishizono H, Miyakawa T, Yachie N, Takao K (2023) Comprehensive behavioral analyses of mice with a glycine receptor alpha 4 deficiency. Mol Brain 16(1):44. https://doi.org/10.1186/s13041-023-01033-x
D’Autréaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8(10):813–824. https://doi.org/10.1038/nrm2256
Del Toro-Barbosa M, Hurtado-Romero A, Garcia-Amezquita LE, García-Cayuela T (2020) Psychobiotics: mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients 12(12):3896. https://doi.org/10.3390/nu12123896
Dinan TG (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74:720–726. https://doi.org/10.1016/j.biopsych.2013.05.001
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Dumoulin A, Triller A, Dieudonné S (2001) IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci 21(16):6045–6057. https://doi.org/10.1523/JNEUROSCI.21-16-06045.2001
Fares J, Bou Diab Z, Nabha S, Fares Y (2019) Neurogenesis in the adult hippocampus: history, regulation, and prospective roles. Int J Neurosci 129(6):598–611. https://doi.org/10.1080/00207454.2018.1545771
Flint AC, Liu X, Kriegstein AR (1998) Nonsynaptic glycine receptor activation during early neocortical development. Neuron 20(1):43–53. https://doi.org/10.1016/s0896-6273(00)80433-x
Forêt S, Maleszka R (2006) Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res 16(11):1404–1413. https://doi.org/10.1101/gr.5075706
Forssten SD, Ouwehand AC, Griffin SM, Patterson E (2022) One giant leap from mouse to man: the microbiota–gut–brain axis in mood disorders and translational challenges moving towards Human Clinical Trials. Nutrients 14(3):568. https://doi.org/10.3390/nu14030568
Giacobini E (1987) Carbonic anhydrase: the first marker of glial development. Curr Top Dev Biol 21:207–215. https://doi.org/10.1016/s0070-2153(08)60138-6
Gomaa EZ (2020) Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113(12):2019–2040. https://doi.org/10.1007/s10482-020-01474-7
Graham JP, Leibler JH, Price LB, Otte JM, Pfeiffer DU, Tiensin T, Silbergeld EK (2008) The animal-human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Rep 123(3):282–299. https://doi.org/10.1177/003335490812300309
Gualtieri P, Marchetti M, Cioccoloni G, De Lorenzo A, Romano L, Cammarano A, Colica C, Condò R, Di Renzo L (2020) Psychobiotics regulate the anxiety symptoms in carriers of allele A of IL-1β gene: a randomized, placebo-controlled clinical trial. Mediators of Inflamm 2346126. https://doi.org/10.1155/2020/2346126
Guo W, Ren D, Zhao L, Jiang F, Song J, Wang X, Kang L (2018) Identification of odorant-binding proteins (OBPs) and functional analysis of phase-related OBPs in the migratory locust. Front Physiol 9:984. https://doi.org/10.3389/fphys.2018.00984
Haas-Neill S, Iwashita E, Dvorkin-Gheva A, Forsythe P (2022) Effects of two distinct psychoactive microbes, Lacticaseibacillus rhamnosus JB-1 and Limosilactobacillus reuteri 6475, on circulating and hippocampal mRNA in male mice. Int J Mol Sci 23:9653. https://doi.org/10.3390/ijms23179653
Håglin L, Domellöf M, Bäckman L, Forsgren L (2020) Low plasma thiamine and phosphate in male patients with Parkinson’s disease is associated with mild cognitive impairment. Clin Nutr ESPEN 37:93–99. https://doi.org/10.1016/j.clnesp.2020.03.012
Hardy H, Harris J, Lyon E, Beal J, Foey AD (2013) Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 5(6):1869–1912. https://doi.org/10.3390/nu5061869
Harvey RJ, Schmieden V, Von Holst A, Laube B, Rohrer H, Betz H (2000) Glycine receptors containing the alpha4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur J Neurosci 12(3):994–1001. https://doi.org/10.1046/j.1460-9568.2000.00993.x
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6(1):39–51. https://doi.org/10.1177/1756283X12459294
Hussaini SMQ, Choi CI, Cho CH, Kim HJ, Jun H, Jang MH (2014) Wnt signaling in neuropsychiatric disorders: ties with adult hippocampal neurogenesis and behavior. Neurosci Biobehav Rev 47:369–383. https://doi.org/10.1016/j.neubiorev.2014.09.005
Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, Wang MX, Sailor KA, Kim JY, Gao Y, Christian KM, Ming GL, Song H (2013a) Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12:215–223. https://doi.org/10.1016/j.stem.2012.11.021
Jang MH, Kitabatake Y, Kang E, Jun H, Pletnikov MV, Christian KM, Hen R, Lucae S, Binder EB, Song H, Ming GI (2013b) Secreted frizzled-related protein 3 (sFRP3) regulates antidepressant responses in mice and humans. Mol Psychiatry 18(9):957–958. https://doi.org/10.1038/mp.2012.158
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96(1):25–34. https://doi.org/10.1016/s0092-8674(00)80956-3
Jonas P, Bischofberger J, Sandkühler J (1998) Corelease of two fast neurotransmitters at a central synapse. Science 281(5375):419–424. https://doi.org/10.1126/science.281.5375.419
Kats IR, Klann E (2019) Translating from cancer to the brain: regulation of protein synthesis by eIF4F. Learn Mem 26(9):332–342. https://doi.org/10.1101/lm.050047.119
Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, Clarke G, Dinan TG (2017) Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 61:50–59. https://doi.org/10.1016/j.bbi.2016.11.018
Knapp LT, Klann E (2002) Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci 22:674–683. https://doi.org/10.1523/JNEUROSCI.22-03-00674.2002
Kochalska K, Oakden W, Słowik T, Chudzik A, Pankowska A, Łazorczyk A, Kozioł P, Andres-Mach M, Pietura R, Rola R, Stanisz GJ, Orzyłowska A (2020) Dietary supplementation with Lactobacillus rhamnosus JB-1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr Res 82:44–57. https://doi.org/10.1016/j.nutres.2020.06.019
Kono Y, Fridovich I (1983) Functional significance of manganese catalase in Lactobacillus plantarum. J Bacteriol 155(2):742–746. https://doi.org/10.1128/jb.155.2.742-746.1983
Kumar A, Pramanik J, Goyal N, Chauhan D, Sivamaruthi BS, Prajapati BG, Chaiyasut C (2023) Gut microbiota in anxiety and depression: unveiling the relationships and management options. Pharmaceuticals 16(4):565. https://doi.org/10.3390/ph16040565
Kuwahara-Otani S, Maeda S, Kobayashi K, Minato Y, Tanaka K, Yamanishi K, Hata M, Li W, Hayakawa T, Noguchi K, Okamura H, Yagi H (2017) Interleukin-18 and its receptor are expressed in gonadotropin-releasing hormone neurons of mouse and rat forebrain. Neurosci Lett 650:33–37. https://doi.org/10.1016/j.neulet.2017.03.051
Lakkis MM, O’Shea KS, Tashian RE (1997) Differential expression of the carbonic anhydrase genes for CA VII (Car7) and CA-RP VIII (Car8) in mouse brain. J Histochem Cytochem 45(5):657–662. https://doi.org/10.1177/002215549704500503
Legendre P (2001) The glycinergic inhibitory synapse. Cell Mol Life Sci 58(5–6):760–793. https://doi.org/10.1007/pl00000899
Lemon N, Canepa E, Ilies MA, Fossati S (2021) Carbonic anhydrases as potential targets against neurovascular unit dysfunction in Alzheimer’s disease and stroke. Front Aging Neurosci 13:772278. https://doi.org/10.3389/fnagi.2021.772278
Li Y, Wu LJ, Legendre P, Xu TL (2003) Asymmetric cross-inhibition between GABAA and glycine receptors in rat spinal dorsal horn neurons. J Biol Chem 278(40):38637–38645. https://doi.org/10.1074/jbc.M303735200
Li D, London SJ, Liu J, Lee W, Jiang X, Van Den Berg D, Bergen AW, Nishita D, Waleh N, Swan GE, Gallaher P, Chou CP, Shih JC, Unger JB, Gauderman WJ, Gilliland F, Johnson CA, Conti DV (2011) Association of the calcyon neuron-specific vesicular protein gene (CALY) with adolescent smoking initiation in China and California. Am J Epidemiol 173(9):1039–1048. https://doi.org/10.1093/aje/kwq471
Lingenfelter BM, Tripurani SK, Tejomurtula J, Smith GW, Yao J (2011) Molecular cloning and expression of bovine nucleoplasmin 2 (NPM2): a maternal effect gene regulated by miR-181a. Reprod Biol Endocrinol 9:40. https://doi.org/10.1186/1477-7827-9-40
Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, Wang S, Tsai YC (2016) Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res 1631:1–12. https://doi.org/10.1016/j.brainres.2015.11.018
Liu D, Ke Z, Luo J (2017) Thiamine deficiency and neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol Neurobiol 54(7):5440–5448. https://doi.org/10.1007/s12035-016-0079-9
Lloyd SE, Grizenkova J, Pota H, Collinge J (2009) Shadoo (Sprn) and prion disease incubation time in mice. Mamm Genome 20(6):367–374. https://doi.org/10.1007/s00335-009-9194-5
Lotrich FE (2015) Inflammatory cytokine-associated depression. Brain Res 1617:113–125. https://doi.org/10.1016/j.brainres.2014.06.032
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Lynch JW (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56(1):303–309. https://doi.org/10.1016/j.neuropharm.2008.07.034
Lyte M (2011) Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 33(8):574–581. https://doi.org/10.1002/bies.201100024
Maeng SH, Hong H (2019) Inflammation as the potential basis in depression. Int Neurourol J 23(2):63–71. https://doi.org/10.5213/inj.1938226.113
Makusheva Y, Chung SH, Akitsu A, Maeda N, Maruhashi T, Ye XQ, Kaifu T, Saijo S, Sun H, Han W, Tang C, Iwakura Y (2022) The C-type lectin receptor Clec1A plays an important role in the development of experimental autoimmune encephalomyelitis by enhancing antigen presenting ability of dendritic cells and inducing inflammatory cytokine IL-17. Exp Anim 71(3):288–304. https://doi.org/10.1538/expanim.21-0191
Manyi-Loh C, Mamphweli S, Meyer E, Okoh A (2018) Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23(4):795. https://doi.org/10.3390/molecules23040795
Mariani E, Polidori MC, Cherubini A, Mecocci P (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Anal Technol Biomed Life Sci 827:65–75. https://doi.org/10.1016/j.jchromb.2005.04.023
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A (2017) Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 7:43859. https://doi.org/10.1038/srep43859
Masuda M, Ide M, Utsumi H, Niiro T, Shimamura Y, Murata M (2012) Production potency of folate, vitamin B(12), and thiamine by lactic acid bacteria isolated from Japanese pickles. Biosci Biotechnol Biochem 76(11):2061–2067. https://doi.org/10.1271/bbb.120414
Matzenbach B, Maulet Y, Sefton L, Courtier B, Avner P, Guénet JL, Betz H (1994) Structural analysis of mouse glycine receptor alpha subunit genes. Identification and chromosomal localization of a novel variant. J Biol Chem 269(4):2607–12
Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM (2011) Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 105:755–764. https://doi.org/10.1017/S0007114510004319
Mindus C, Ellis J, van Staaveren N, Harlander-Matauschek A (2021) Lactobacillus-based probiotics reduce the adverse effects of stress in rodents: a meta-analysis. Front Behav Neurosci 15:642757. https://doi.org/10.3389/fnbeh.2021.642757
Mitrea L, Nemeş SA, Szabo K, Teleky BE, Vodnar DC (2022) Guts imbalance imbalances the brain: a review of gut microbiota association with neurological and psychiatric disorders. Front Med 9:813204. https://doi.org/10.3389/fmed.2022.813204
Miyamoto H, Shimohata A, Abe M, Abe T, Mazaki E, Amano K, Suzuki T, Tatsukawa T, Itohara S, Sakimura K, Yamakawa K (2017) Potentiation of excitatory synaptic transmission ameliorates aggression in mice with Stxbp1 haploinsufficiency. Hum Mol Genet 26(24):4961–4974. https://doi.org/10.1093/hmg/ddx379
Mkrtchyan G, Aleshin V, Parkhomenko Y, Kaehne T, Di Salvo ML, Parroni A, Contestabile R, Vovk A, Bettendorff L, Bunik V (2015) Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis. Sci Rep 5:12583. https://doi.org/10.1038/srep12583
Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S (2005) Klaenhammer TR (2005) Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA 102(8):2880–2885. https://doi.org/10.1073/pnas.0500098102
Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF (2016) Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther 22(2):102–117. https://doi.org/10.1111/cns.12490
Mori M, Gähwiler BH, Gerber U (2002) Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol 539(Pt 1):191–200. https://doi.org/10.1113/jphysiol.2001.013147
Mörkl S, Butler MI, Holl A, Cryan JF, Dinan TG (2020) Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr Nutr Rep 9(3):171–182. https://doi.org/10.1007/s13668-020-00313-5
Musso G, Gambino R, Cassader M (2010) Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care 33(10):2277–2284. https://doi.org/10.2337/dc10-0556
Muthusamy N, Faundez V, Bergson C (2012) Calcyon, a mammalian specific NEEP21 family member, interacts with adaptor protein complex 3 (AP-3) and regulates targeting of AP-3 cargoes. J Neurochem 123(1):60–72. https://doi.org/10.1111/j.1471-4159.2012.07814.x
Nicoll RA, Malenka RC, Kauer JA (1990) Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev 70(2):513–565. https://doi.org/10.1152/physrev.1990.70.2.513
Olsen RH, Johnson LA, Zuloaga DG, Limoli CL, Raber J (2013) Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem 125(2):303–313. https://doi.org/10.1111/jnc.12187
Oroojzadeh P, Bostanabad SY, Lotfi H (2022) Psychobiotics: the influence of gut microbiota on the gut-brain axis in neurological disorders. J Mol Neurosci 72:1952–1964. https://doi.org/10.1007/s12031-022-02053-3
Passet B, Castille J, Makhzami S, Truchet S, Vaiman A, Floriot S, Moazami-Goudarzi K, Vilotte M, Gaillard AL, Helary L, Bertaud M, Andréoletti O, Vaiman D, Calvel P, Daniel-Carlier N, Moudjou M, Beauvallet C, Benharouga M, Laloé D, Mouillet-Richard S, Duchesne A, Béringue V, Vilotte JL (2020) The Prion-like protein Shadoo is involved in mouse embryonic and mammary development and differentiation. Sci Rep 10(1):6765. https://doi.org/10.1038/s41598-020-63805-y
Patapoutian A, Reichardt LF (2000) Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol 10(3):392–399. https://doi.org/10.1016/s0959-4388(00)00100-8
Pećina-Šlaus N, Kafka A, Varošanec AM, Marković L, Krsnik Ž, Njirić N, Mrak G (2016) Expression patterns of Wnt signaling component, secreted frizzled-related protein 3 in astrocytoma and glioblastoma. Mol Med Rep 13(5):4245–4251. https://doi.org/10.3892/mmr.2016.5061
Pelosi P, Calvello M, Ban L, Calvello M, Ban L, Ban L (2005) Diversity of odorant-binding proteins and chemosensory proteins in insects. Chem Senses 30(1):291–292. https://doi.org/10.1093/chemse/bjh229
Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, Carter DR (2011) Pseudogenes: pseudo-functional or key regulators in health and disease? RNA 17(5):792–798. https://doi.org/10.1261/rna.2658311
Poluektova E, Yunes R, Danilenko V (2021) The putative antidepressant mechanisms of probiotic bacteria: relevant genes and proteins. Nutrients 13(5):1591. https://doi.org/10.3390/nu13051591
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16(6):341–352. https://doi.org/10.1038/nri.2016.42
Russier M, Kopysova IL, Ankri N, Ferrand N, Debanne D (2002) GABA and glycine co-release optimizes functional inhibition in rat brainstem motoneurons in vitro. J Physiol 541(Pt 1):123–137. https://doi.org/10.1113/jphysiol.2001.016063
Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, Uruno K, Kumada S, Nishiyama K, Nishimura A, Okada I, Yoshimura Y, Hirai S, Kumada T, Hayasaka K, Fukuda A, Ogata K, Matsumoto N (2008) De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet 40:782–788. https://doi.org/10.1038/ng.150
Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ (2016) Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci 39:763–781. https://doi.org/10.1016/j.tins.2016.09.002
Scaglione CN, Xu Q, Ramanujan VK (2016) Direct measurement of catalase activity in living cells and tissue biopsies. Biochem Biophys Res Commun 470(1):192–196. https://doi.org/10.1016/j.bbrc.2016.01.026
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):453–462. https://doi.org/10.1016/j.cub.2014.03.034
Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74(1):1–58. https://doi.org/10.1016/j.pneurobio.2004.05.006
Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A (2013) Loss of dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 12:204–214. https://doi.org/10.1016/j.stem.2012.11.010
Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90(3):859–904. https://doi.org/10.1152/physrev.00045.2009
Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3(4):431–443. https://doi.org/10.1016/j.arr.2004.05.002
Shen C, Rathore SS, Yu H, Gulbranson DR, Hua R, Zhang C, Schoppa NE, Shen J (2015) The trans-SNARE-regulating function of Munc18-1 is essential to synaptic exocytosis. Nat Commun 6:8852. https://doi.org/10.1038/ncomms9852
Shohayeb B, Diab M, Ahmed M, Ng DCH (2018) Factors that influence adult neurogenesis as potential therapy. Transl Neurodegener 7:4. https://doi.org/10.1186/s40035-018-0109-9
Shu Y, Xiang M, Zhang P, Qi G, He F, Zhang Q, Zhang Z, Lv Z, Peng X, Cai H, Tian B (2018) Wnt-5a promotes neural development and differentiation by regulating CDK5 via Ca2+/Calpain pathway. Cell Physiol Biochem 51:2604–2615. https://doi.org/10.1159/000495932
Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA (2004) Analysis of the set of GABA(A) receptor genes in the human genome. J Biol Chem 279(40):41422–41435. https://doi.org/10.1074/jbc.M401354200
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24(8):1583. https://doi.org/10.3390/molecules24081583
Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, Hannon GJ (2008) Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453(7194):534–538. https://doi.org/10.1038/nature06904
Tcatchoff L, Nespoulous C, Pernollet JC, Briand L (2006) A single lysyl residue defines the binding specificity of a human odorant-binding protein for aldehydes. FEBS Lett 580(8):2102–2108. https://doi.org/10.1016/j.febslet.2006.03.017
Teran MDM, de Moreno de LeBlanc A, Savoy de Giori G, LeBlanc JG (2021) Thiamine-producing lactic acid bacteria and their potential use in the prevention of neurodegenerative diseases. Appl Microbiol Biotechnol 105(5):2097–2107. https://doi.org/10.1007/s00253-021-11148-7
Wang Y, Kasper LH (2014) The role of microbiome in central nervous system disorders. Brain Behav Immun 38:1–12. https://doi.org/10.1016/j.bbi.2013.12.015
Wang W, Li S, Dong HP, Lv S, Tang YY (2009) Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 85(3–4):127–135. https://doi.org/10.1016/j.lfs.2009.05.003
Wang X, Zhang P, Zhang X (2021) Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules 26(19):6076. https://doi.org/10.3390/molecules26196076
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5(1):51–66. https://doi.org/10.1038/nprot.2009.197
Whitfield KC, Bourassa MW, Adamolekun B, Bergeron G, Bettendorff L, Brown KH, Cox L, Fattal-Valevski A, Fischer PR, Frank EL, Hiffler L, Hlaing LM, Jefferds ME, Kapner H, Kounnavong S, Mousavi MPS, Roth DE, Tsaloglou MN, Wieringa F, Combs GF Jr (2018) Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci 1430(1):3–43. https://doi.org/10.1111/nyas.13919
Wiśniewski JR, Gaugaz FZ (2015) Fast and sensitive total protein and peptide assays for proteomic analysis. Anal Chem 87:4110–4116. https://doi.org/10.1021/ac504689z
Xiao J, Dai R, Negyessy L, Bergson C (2006) Calcyon, a novel partner of clathrin light chain, stimulates clathrin-mediated endocytosis. J Biol Chem 281(22):15182–15193. https://doi.org/10.1074/jbc.M600265200
Xu M, Tian P, Zhu H, Zou R, Zhao J, Zhang H, Wang G, Chen W (2022) Lactobacillus paracasei CCFM1229 and Lactobacillus rhamnosus CCFM1228 alleviated depression- and anxiety-related symptoms of chronic stress-induced depression in mice by regulating xanthine oxidase activity in the brain. Nutrients 14(6):1294. https://doi.org/10.3390/nu14061294
Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O (2005) Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21(5):650–659. https://doi.org/10.1093/bioinformatics/bti042
Yunes RA, Poluektova EU, Dyachkova MS, Klimina KM, Kovtun AS, Averina OV, Orlova VS, Danilenko VN (2016) GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42:197–204. https://doi.org/10.1016/j.anaerobe.2016.10.011
Yunes RA, Poluektova EU, Vasileva EV, Odorskaya MV, Marsova MV, Kovalev GI, Danilenko VN (2020) A multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob Proteins 12(3):973–979. https://doi.org/10.1007/s12602-019-09601-1
Zhang CX, Wang HY, Chen TX (2019) Interactions between intestinal microflora/probiotics and the immune system. Biomed Res Int 2019:6764919. https://doi.org/10.1155/2019/6764919
Zhao Y, Wu Y, Hu H, Cai J, Ning M, Ni X, Zhong C (2014) Downregulation of transketolase activity is related to inhibition of hippocampal progenitor cell proliferation induced by thiamine deficiency. Biomed Res Int 2014:572915. https://doi.org/10.1155/2014/572915
Zhu X, Han Y, Du J, Liu R, Jin K, Yi W (2017) Microbiota-gut-brain axis and the central nervous system. Oncotarget 8(32):53829–38. https://doi.org/10.18632/oncotarget.17754
Zielińska D, Karbowiak M, Brzezicka A (2022) The role of psychobiotics to ensure mental health during the COVID-19 pandemic-a current state of knowledge. Int J Environ Res Public Health 19(17):11022. https://doi.org/10.3390/ijerph191711022
Zoghbi HY, Bear MF (2012) Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol 4(3):a009886. https://doi.org/10.1101/cshperspect.a009886
Acknowledgements
The authors wish to thank Prof. Greg J. Stanisz (Department of Medical Biophysics, University of Toronto, ON, Canada) and SANPROBI Sp. z o.o., Sp. k. (Szczecin, Poland) for a generous gift of Lactobacillus rhamnosus JB-1 and Bifidobacterium longum Rosell®-175 strains, respectively.
Funding
This research was funded by the Association of Lublin Universities in Lublin, Poland, project No. ZUL/INT/2/2023/WET. Interproject program no. INT/002/2022/I-N.
Author information
Authors and Affiliations
Contributions
ŁSJ, KS, and PW designed the experiment. KS performed oral gavages. PW and KS collected samples. AW and MM performed the microbe isolation, cultivation, and microbial strains preservation. KM, AC, and ŁSJ performed laboratory analyses. KM and AC performed all the statistical analysis and figures generation. ŁSJ, AM, and ZG wrote the manuscript. PW gave suggestions for manuscript revision. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures were approved by The Local Ethics Committee on Animal Experimentation of the University of Life Sciences in Lublin, Poland (license no 65/2022). Housing and experimental procedures were performed in accordance with the EU Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. Throughout the experimental period, the health status of mouse was regularly monitored by a veterinarian.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jarosz, Ł.S., Socała, K., Michalak, K. et al. The effect of psychoactive bacteria, Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1, on brain proteome profiles in mice. Psychopharmacology 241, 925–945 (2024). https://doi.org/10.1007/s00213-023-06519-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06519-z