Abstract
Rationale
Inadequate responses to current schizophrenia treatments have accelerated research into novel therapeutic approaches.
Objectives
This study investigated the efficacy and tolerability of adjunctive L-theanine, an ingredient with neuroimmunomodulatory and neuroprotective properties, for chronic schizophrenia.
Methods
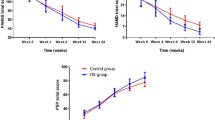
Eighty chronic schizophrenia inpatients were equally assigned to receive risperidone (6 mg/day) plus either L-theanine (400 mg/day) or matched placebo in this 8-week, randomized, parallel-group, double-blind, placebo-controlled trial. The participants were assessed using the Positive and Negative Syndrome Scale (PANSS) by recording the results of subscales at baseline and weeks 4 and 8 to measure treatment efficacy. Additionally, the participants were assessed for the Hamilton Depression Rating Scale (HDRS) and adverse events, including the Extrapyramidal Symptom Rating Scale (ESRS).
Results
Sixty patients, 30 in each group, were included in the analyses. All baseline demographic and clinical characteristics were comparable between the groups (p-values > 0.05). The reduction rates from baseline to endpoint in negative, general psychopathology, and total scores of PANSS were greater in the L-theanine group (p-values = 0.03, 0.01, and 0.04, respectively). Regarding general psychopathology scores, the reduction in the L-theanine group was also greater until week 4 (p-value < 0.01). The time × treatment interaction effect was significant on negative (p-value = 0.03), general psychopathology (p-value < 0.01), and total (p-value = 0.04) scores of PANSS, indicating additional improvements in the L-theanine group. The HDRS and side effects were comparable between the groups (p-values > 0.05).
Conclusions
L-Theanine adjunct to risperidone safely and tolerably outperformed adjunctive placebo for schizophrenia, and promising evidence indicated its effects on primary negative symptoms, which need to be scrutinized in further studies.
Trial registration
The study protocol was registered and published prospectively in the Iranian Registry of Clinical Trials (http://www.irct.ir; registration number: IRCT20090117001556N133) on 2020–12-12.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- DSM-V:
-
Diagnostic and Statistical Manual of Mental Disorders, fifth edition
- PANSS:
-
Positive and Negative Syndrome Scale
- SD:
-
Standard deviation
- HDRS:
-
Hamilton Depression Rating Scale
- ESRS:
-
Extrapyramidal Symptom Rating Scale
- MD:
-
Mean difference
- CI:
-
Confidence interval
- GLM:
-
General linear model
- ANOVA:
-
Analysis of variance
References
Akhondzadeh S (2001) The 5-HT hypothesis of schizophrenia. Idrugs 4(3):295–300
Akhondzadeh S, Mohammadi MR, Amini-Nooshabadi H, Davari-Ashtiani R (1999) Cyproheptadine in treatment of chronic schizophrenia:a double-blind, placebo-controlled study. J Clin Pharm Ther 24:49–52
Association WM (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T (2014) The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry 5:47
Chouinard G, Margolese HC (2005) Manual for the extrapyramidal symptom rating scale (ESRS). Schizophr Res 76:247–265
Collaborators GMD (2022) Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Psychiatry 9:137–150
Deb S, Dutta A, Phukan BC, Manivasagam T, Justin Thenmozhi A, Bhattacharya P, Paul R, Borah A (2019) Neuroprotective attributes of L-theanine, a bioactive amino acid of tea, and its potential role in Parkinson’s disease therapeutics. Neurochem Int 129:104478
First MB, Williams JB, Karg RS, Spitzer RL (2015) Structured clinical interview for DSM-5—research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: Am Psychiatric Ass 2015:1–94
Food and Drug Administration (2006) (FDA) CFSAN/Office of Food Additive Safety. Agency Response Letter GRAS Notice No. GRN 000209. GRAS Notice Inventory of L-Theanine
Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P (2015) Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull 41:892–899
Hagihara R, Ohno S, Hayashi M, Tabata K, Endo H (2021) Production of L-theanine by Escherichia coli in the absence of supplemental ethylamine. Appl Environ Microbiol 87:e00031–21. https://doi.org/10.1128/aem.00031-21
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hengartner MP, Plöderl M (2018) Statistically significant antidepressant-placebo differences on subjective symptom-rating scales do not prove that the drugs work: effect size and method bias matter! Front Psychiatry 9:517
Johns CA, Thompson JW (1995) Adjunctive treatments in schizophrenia: pharmacotherapies and electroconvulsive therapy. Schizophr Bull 21:607–619
Jönsson AK, Schill J, Olsson H, Spigset O, Hägg S (2018) Venous thromboembolism during treatment with antipsychotics: a review of current evidence. CNS Drugs 32:47–64
Kadakia A, Catillon M, Fan Q, Williams GR, Marden JR, Anderson A, Kirson N, Dembek C (2022) The Economic burden of schizophrenia in the United States. J Clin Psychiatry 83:22m14458. https://www.psychiatrist.com/jcp/schizophrenia/economic-burden-schizophrenia-united-states/?trk=organization_guest_main-feed-card_feed-article-content
Kakuda T, Nozawa A, Sugimoto A, Niino H (2002) Inhibition by theanine of binding of [3H] AMPA,[3H] kainate, and [3H] MDL 105,519 to glutamate receptors. Biosci Biotechnol Biochem 66:2683–2686
Kashani L, Eslatmanesh S, Saedi N, Niroomand N, Ebrahimi M, Hosseinian M, Foroughifar T, Salimi S, Akhondzadeh S (2017) Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry 50(2):64–68
Katasonov AB (2018) Neurobiological effects of theanine and its possible use in neurology and psychiatry. Zh Nevrol Psikhiatr Im S S Korsakova 118:118–124
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Kianimehr G, Fatehi F, Hashempoor S, Khodaei-Ardakani M-R, Rezaei F, Nazari A, Kashani L, Akhondzadeh S (2014) Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. DARU J Pharmaceutical Sci 22:55
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863
Lardner AL (2014) Neurobiological effects of the green tea constituent theanine and its potential role in the treatment of psychiatric and neurodegenerative disorders. Nutr Neurosci 17:145–155
Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S (2007) Defining ‘response’in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacol 32:1903–1910
Medscape® (2017) Multi-Drug Interaction Checker. Medscape New York. https://reference.medscape.com/drug-interactionchecker
Moncrieff J, Kirsch I (2015) Empirically derived criteria cast doubt on the clinical significance of antidepressant-placebo differences. Contemp Clin Trials 43:60–62
National Institute of Mental Health, 2022. Schizophrenia. U.S. Department of Health and Human Services, National Institutes of Health. Retrieved March 19, 2023, from https://www.nimh.nih.gov/health/statistics/schizophrenia
Ota M, Wakabayashi C, Sato N, Hori H, Hattori K, Teraishi T, Ozawa H, Okubo T, Kunugi H (2015) Effect of L-theanine on glutamatergic function in patients with schizophrenia. Acta Neuropsychiatrica 27:291–296
Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia Lancet 388:86–97
Peralta V, Cuesta MJ, Martinez-Larrea A, Serrano JF (2000) Differentiating primary from secondary negative symptoms in schizophrenia: a study of neuroleptic-naive patients before and after treatment. Am J Psychiatry 157:1461–1466
Potkin SG, Kane JM, Correll CU, Lindenmayer J-P, Agid O, Marder SR, Olfson M, Howes OD (2020) The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. npj Schizophrenia 6: 1. https://www.nature.com/articles/s41537-019-0090-z
Sakamoto FL, Ribeiro RMP, Bueno AA, Santos HO (2019) Psychotropic effects of L-theanine and its clinical properties: from the management of anxiety and stress to a potential use in schizophrenia. Pharmacol Res 147:104395
Salehi A, Namaei P, TaghaviZanjani F, Bagheri S, Moradi K, Khodaei Ardakani M-R, Akhondzadeh S (2022) Adjuvant palmitoylethanolamide therapy with risperidone improves negative symptoms in patients with schizophrenia: a randomized, double-blinded, placebo-controlled trial. Psychiatry Res 316:114737
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 1:100–107
Stern W (1949) The intelligence quotient readings in general psychology. Prentice-Hall Inc, New York, NY, US, pp 338–341
Takeshima M, Miyazaki I, Murakami S, Kita T, Asanuma M (2016) L-Theanine protects against excess dopamine-induced neurotoxicity in the presence of astrocytes. J Clin Biochem Nutrition 59:93–99
Türközü D, Şanlier N (2017) L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit Rev Food Sci Nutr 57:1681–1687
Wakabayashi C, Numakawa T, Ninomiya M, Chiba S, Kunugi H (2012) Behavioral and molecular evidence for psychotropic effects in L-theanine. Psychopharmacology 219:1099–1109
Wang L, Brennan M, Li S, Zhao H, Lange KW, Brennan C (2022) How does the tea L-theanine buffer stress and anxiety. Food Sci Human Wellness 11:467–475
Wang Q, Zheng Y, Ho C-T, Huang J, Guan X, Lai C, Gao H, Lin B (2021) L-theanine as a promising agent on brain health-promoting foods–a review. Journal of Food Bioactives 13. http://www.isnff-jfb.com/index.php/JFB/article/view/200
Williams J, Sergi D, McKune AJ, Georgousopoulou EN, Mellor DD, Naumovski N (2019) The beneficial health effects of green tea amino acid l-theanine in animal models: promises and prospects for human trials. Phytother Res 33:571–583
Acknowledgements
This study was performed in support of Dr. Setareh Fattollahzadeh-Noor’s postgraduate thesis toward the Iranian Board of Psychiatry.
Funding
This study was supported by a grant from the Tehran University of Medical Sciences (TUMS) to Professor Shahin Akhondzadeh (grant number 49661). TUMS had no role in the design, conduct, data collection, analysis, data interpretation, manuscript preparation, review, final approval, and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
SA and MK: conceptualization, project administration, supervision, funding acquisition, methodology, and software; AS, SF, BF, and FAB: writing—original draft, formal analysis, editing, data curation, and investigation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol followed the ethical principles of the seventh revision of the Declaration of Helsinki, as revised in Brazil in 2013, and was approved by the institutional research ethics committee on 2020–09-22 (approval code: IR.TUMS.DDRI.REC.1399.034).
Consent to participate
Informed consent was obtained from all patients or their legally authorized representatives while being aware of the possibility of withdrawing from the study at any time without affecting their therapy and relationship with healthcare providers.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shamabadi, A., Fattollahzadeh-Noor, S., Fallahpour, B. et al. L-Theanine adjunct to risperidone in the treatment of chronic schizophrenia inpatients: a randomized, double-blind, placebo-controlled clinical trial. Psychopharmacology 240, 2631–2640 (2023). https://doi.org/10.1007/s00213-023-06458-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06458-9