Abstract
Objective
Despite advances in pharmacology, the treatment of schizophrenia (SZ) remains a challenge due to relapse after antipsychotic discontinuation and multiple adverse effects of antipsychotics. We hypothesized that a low dose of risperidone in combination with sertraline would reduce serious adverse effects without decreasing treatment response. This study aimed to examine the efficacy, safety, and tolerability of low-dose risperidone combined with sertraline to reduce risperidone dose and serious adverse effects in first-episode and medication-naive (FEMN) SZ patients.
Methods
A total of 230 patients with FEMN SZ were randomly assigned to receive low-dose risperidone in combination with sertraline (RS group) or regular-dose risperidone (control group). The Positive and Negative Syndrome Scale (PANSS), Hamilton Depression Rating Scale (HAMD), and Personal and Social Performance Scale (PSP) were assessed at baseline and the end of the first, second, third, and sixth months. In addition, serum prolactin levels and extrapyramidal symptoms were measured at baseline and follow-up.
Results
Repeated measures ANCOVA showed significant interaction effects of treatment by time on psychotic symptoms, as well as HAMD, PSP scores, prolactin levels, and extrapyramidal symptoms (all p < 0.05). Compared with the control group, the RS group had greater decreases in PANSS total score and its subscores and HAMD score (all p < 0.01) and a greater increase in PSP total score (p < 0.01). Notably, side effects were lower in the RS group relative to the control group. Improvements in HAMD and PANSS total scores, changes in prolactin levels and gender predicted improvements in PSP from baseline to month 6.
Conclusions
Our study suggests that low-dose risperidone in combination with sertraline was more effective for psychotic symptoms and psychosocial functioning, with significantly fewer adverse effects in patients with FEMN SZ.
Trial registration number: ClinicalTrials.gov, NCT04076371
Similar content being viewed by others
Introduction
Schizophrenia (SZ) is a severe, chronic mental disorder with a significant decline in psychosocial functioning [1]. Patients with SZ usually present with psychotic symptoms and chronic decline in cognitive functioning as well as emotional disturbances. The etiology of SZ is unknown, with a high rate of relapse and unresponsiveness to antipsychotics, resulting in family burden and family distress [2]. Currently, atypical antipsychotics are commonly prescribed to alleviate psychotic symptoms in SZ [3]. However, the efficacy of antipsychotic medications does not achieve satisfactory clinical response, and it is estimated that approximately 30% of patients with SZ exhibit non-response to antipsychotic medications [4, 5]. In particular, patients with SZ usually present with negative symptoms, which remains a key treatment challenge and a major target for novel treatment strategies [6].
Risperidone, one of the atypical antipsychotics used to treat SZ, is recommended as a first-line agent and is an effective treatment strategy to alleviate acute and chronic psychosis in patients with SZ at admission [7,8,9]. Previous large studies of relapse in patients treated with risperidone or haloperidol found a significantly lower relapse rate in the risperidone group [10]. In another meta-analysis, risperidone, amisulpride, and olanzapine were shown to be more effective than other antipsychotics in SZ patients [11]. Atypical antipsychotics cause different degrees and types of side effects due to different affinities for different receptors, and recent studies comparing the adverse effects of different antipsychotics on SZ have confirmed that antipsychotic-induced side effects differ significantly among patients with first-episode psychosis [12,13,14]. Risperidone ranks high in the list of antipsychotics associated with significantly elevated prolactin levels [11]. In addition, risperidone is associated with extrapyramidal symptoms and a high rate of antiparkinson medication use in patients with SZ, although a lower rate compared to haloperidol [15, 16]. Based on the CATIE study, it was reported that risperidone may cause akathisia in 7% of SZ patients [17].
Risperidone can drastically improve clinical symptoms in patients with SZ, but its role is limited due to the high incidence of hyperprolactinemia and extrapyramidal adverse effects. Exploring new combinations of antipsychotics to improve efficacy and reduce side effects in the treatment of SZ is an interesting topic. To reduce the adverse effects of risperidone, low doses of risperidone can be helpful for patients with SZ. However, there is a need to investigate a new clinical treatment option with fewer adverse effects and better efficacy than standard doses of risperidone. Given the negative symptoms and impairment of social functioning in SZ patients, we chose to use a selective 5-hydroxytryptamine reuptake inhibitor (SSRI) in combination with low-dose risperidone to alleviate symptoms in SZ patients. SSRIs have selective and potent inhibitory effects on presynaptic 5-HT reuptake and increase the 5-HT concentration in the synaptic gap, thereby promoting the action of the 5-hydroxytryptamine system. Importantly, sertraline has been reported to be the most potent SSRI in increasing extracellular DA concentrations in the striatum, and thus improving negative symptoms [18, 19]. Therefore, the combination with risperidone has the potential to alleviate negative symptoms and social functioning in SZ patients [20]. Sertraline is currently approved by the US Food and Drug Administration for the treatment of obsessive–compulsive disorder, self-injury, aggression, and depression in children aged 6 to 17 years [21]. Furthermore, it has a low activating effect compared to other psychotropic drugs and minimal metabolic interactions with other drugs compared to other SSRIs.
In the present study, we hypothesized that a low daily dose of risperidone combined with sertraline would be superior to standard doses of risperidone (4–6 mg/day) in improving psychosocial functioning and reducing adverse effects in FEMN SZ patients. Although risperidone can be utilized up to 16 mg/day, the purpose of this study was to investigate whether low-dose combination therapy would improve negative symptoms and social functioning, and reduce side effects more than the recommended dose of risperidone. Therefore, the manufacture’s recommended dose of risperidone (4–6 mg/day) was chosen for patients in the control group. In this 24 week open-label study, we investigated the following questions in patients with FEMN SZ: (1) to compare the improvement in psychotic symptoms in the low-dose risperidone combined with sertraline group (RS) and the risperidone monotherapy group (control group); (2) to compare the improvement in psychosocial functioning in the RS and control groups; (3) to compare the two groups in terms of improvements in depression and anxiety symptoms; (4) to compare the adverse effects of the two groups; and (5) is improvement in psychosocial functioning associated with a reduction in psychotic symptoms or adverse effects?
Methods
Subjects
This study was conducted at First Hospital of Shanxi Medical University. The study procedures and protocol were approved by the Ethics Committee of First Hospital of Shanxi Medical University and all participants or their guardians signed written informed consent.
A total of 263 patients from First Hospital of Shanxi Medical University were screened. Eligible adult patients (18–45 years) with SZ diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and Structured Clinical Interview for DSM Disorders (SCID) were recruited. All patients were first-episode medication-naïve patients (FEMN) with SZ. The complete eligibility criteria were shown as follows: (1) male or female outpatients; (2) 18–45 years of age; (3) disease duration of SZ not exceeding 60 months; (4) antipsychotic naïve; and (5) more than 9 years of education. Exclusion criteria were: (1) substance dependence except for nicotine; (2) major somatic comorbidities; and (3) abnormal values on routine biochemical tests.
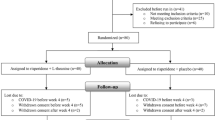
Thirty-three patients were excluded and 230 patients were randomized to two treatment groups (115 in each group). The reasons for the exclusion included ineligible (n = 10), not meeting inclusion criteria (n = 9) and refusal to participate (n = 14).
Study design
This was a 24 week, randomized, controlled open-label study. Patients were randomly assigned to receive low-dose risperidone (2–3.5 mg/day) and sertraline (50–100 mg/day) in the RS group (n = 109 patients) or normal-dose risperidone (4–6 mg/day) monotherapy in the control group (n = 89 patients) (Additional file 1: Fig. S1). First, a computer generated a sequence of random numbers. Then, an independent third party randomized patients to the RS and control groups based on their assigned computer-generated sequence of numbers. Interviewers remained blinded to treatment assignment throughout the trial. Patients were followed up for 24 weeks. The trial consisted of five visits, conducted at baseline, month 1, month 2, month 3, and month 6. Nurses ensured treatment adherence during the study period.
Recruited participants were allowed to take clonazepam (2–3 mg/d) during the early phase of treatment to treat sleep problems and relieve anxiety symptoms and acute agitation. Past medical history, ECG, and physical examination reports were obtained from all participants or their relatives.
Treatment outcomes
The main outcome was clinical symptoms assessed by PANSS [22]. The endpoint was the changes from baseline PANSS scores to the value in the sixth month. Secondary efficacy outcomes were depressive symptoms as evaluated by the Hamilton Depression Rating Scale (HAMD) [23], psychosocial functioning as evaluated by the Personal and Social Performance Scale (PSP) [24], and CGI-S [25], and extrapyramidal symptoms (EPS) assessed by Extrapyramidal Symptom Rating Scale (ESRS) [26]. These outcomes were assessed at baseline, at the end of months 1, 2, 3, and 6.
In addition, blood prolactin levels were determined in the hospital laboratory using commercially available kits.
Statistical analysis
Required sample sizes for adequate power were calculated using the G*Power program. Based on a one-sided α of 0.05, a sample size of 102 evaluable patients in each treatment group was needed to identify the effect size of 0.35 at 80% power. Assuming a dropout rate of 10%, a minimum sample of 204 evaluable patients (102 patients in each group) was needed.
Data missing for patients who dropped out were imputed via the last observation carried forward (LOCF) method. All analyses to evaluate between-group differences were conducted for an intent to treat (ITT) analysis set that included all the patients who received treatment. Demographic characteristics, baseline psychiatric symptoms, depressive symptoms, and psychosocial functioning between the RS and control groups were analyzed by one-way analysis of variance (ANOVA).
A repeated measures multivariate analysis of variance (RM-MANOVA) was used to compare the efficacy of the two treatment regimens. For dependent variables, prolactin levels and symptom scores measured at 5 time points were considered as repeated measures within effects, treatment groups (RS and control groups) were considered as between effects, and age, gender, age at onset, and education were covariates. In each RM-ANOVA model, the independent variables were outcome measures over time. If significant differences were observed in the interaction effect of the treatment group by time, an analysis of covariance (ANCOVA) with baseline value as covariates were conducted to examine differences in symptom scores assessed at different time points (months 1, 2, 3, and 6). In addition, ANCOVA was performed to compare the changes in symptom scores from baseline to follow-up.
Linear regression analysis was performed to identify variables associated with improvement in primary or secondary outcome measures. The following variables were potentially associated with the outcome measure and were therefore added to the regression model: baseline symptoms, gender, age, age at onset, duration of illness, and education.
Data were analyzed using PASW Statistics (SPSS 22.0, Inc., Chicago). Significance was set at p < 0.05 for all analyses. Given the multiple comparisons in the multivariate analysis, Bonferroni’s correction was used.
Results
Baseline characteristics
Eligible patients were randomized to the RS group (n = 115) and the control group (n = 115). A total of 32 patients dropped out in the follow-up (n = 6 in the RS group and n = 26 in the control group). In the RS group, 9 patients discontinued for side effects caused by antipsychotics and 3 patients were lost to follow-up. In the control group, 21 patients discontinued for side effects caused by antipsychotics and 5 patient was lost to follow-up. The rate of dropout was higher in the control group (n = 26) than in the RS group (n = 6) (Fig. 1). A total of 230 patients were included in the efficacy ITT-population analysis.
At baseline, there were no significant differences between the RS and control groups in terms of age, gender, duration of disease, age at onset, and education (all p > 0.05). In addition, there were no significant differences in PANSS scores, CGI-S, PSP, and HAMD total scores between the two groups (all p > 0.05). In addition, there was also no difference in prolactin levels between the RS and control groups (p > 0.05).
At baseline, correlation analysis showed that serum prolactin levels were negatively correlated with the duration of disease in SZ patients (r = − 0.18, p = 0.008). PANSS total score was positively correlated with HAMD, and CGI-S scores (all p < 0.001) and negatively correlated with PSP total score (r = − 0.60, p < 0.001). In addition, PANSS total score was associated with age, gender, and duration of disease (all p < 0.05). Therefore, age, gender and age of onset were added as covariates in the following analysis (Table 1).
Primary outcomes
RM-MANCOVA revealed a significant group-by-time interaction effect on PANSS scores, controlling for age, years of education, gender, and age at onset (Wilks’ lambda F = 55.4, p < 0.001) (Fig. 1). In univariate RM-ANCOVA analysis, significant group-by-time interaction effects for PANSS total score (F = 39.2, p < 0.001) and its 3 subscores (all p < 0.01) (Table 2) (Bonferroni Corrected p < 0.01).
The ANCOVA analysis also showed that after 6 months of treatment, the RS group had greater improvements in the PANSS total score and its subscores after controlling for baseline values (all p < 0.01). After covarying for covariates, we found that the PANSS total scores were significantly lower in the RS group than in the control group (p < 0.01).
Secondary outcomes
We found significant group-by-time interaction effects for CGI-S, PSP and HAMD scores, as well as prolactin levels and ESRS total score (all p < 0.05) (Tables 2, 3) (Fig. 2) (Bonferroni Corrected p < 0.01 for prolactin levels and Bonferroni Corrected p > 0.05 for ESRS total score). After controlling for the baseline values, there were significant differences between the RS and control groups in the changes in CGI-S, PSP, HAMD total scores, prolactin levels, and ESRS total score. At the 6 month follow-up, the RS group had significantly higher PSP total scores and lower HAMD total scores and prolactin levels than the control group (all p < 0.05).
However, there was a positive correlation between the decrease in CGI-S and the increase in PSP total score (r = 0.83, p < 0.001), and between the increase in PSP total score and the decrease in PANSS and HAMD total scores (both p < 0.01). Interestingly, we also found significant associations between an increase in prolactin levels and decreases in PANSS, HAMD, or an increase in total PSP scores (all p < 0.05). We added age, gender, duration of illness, baseline PSP and decreases in PANSS, HAMD, and CGI-S scores as covariates in our regression model and found that decrease in HAMD and PANSS total scores, change in prolactin levels and gender were predictors of improvement in PSP (R2 = 0.64).
In addition, patients in the RS group had fewer extrapyramidal symptoms and used less benzhexol compared to controls (Table 3).
Discussion
The main findings of this study were that (1) after 6 months of treatment, low-dose risperidone in combination with sertraline was more efficacious than standard-dose risperidone alone in terms of psychotic symptoms and depressive symptoms; (2) the RS group was more efficacious than the control group in terms of improvement in psychosocial functioning; (3) the RS group had fewer adverse effects than the control group, including prolactin levels and EPS symptoms; and (4) improvement in psychosocial functioning was associated with changes in clinical symptoms and prolactin levels.
We found that low-dose risperidone combined with sertraline treatment for 24 weeks was more effective than risperidone monotherapy in alleviating negative symptoms, improving social functioning and reducing side effects in patients with FEMN SZ. That is, 24 weeks of low-dose risperidone combined with sertraline not only improved the efficacy in patients with FEMN SZ but also reduced adverse effects, such as elevated EPS and serum prolactin, thereby improving the safety of risperidone in FEMN patients. Furthermore, in line with other studies, the addition of sertraline was effective in improving depressive symptoms in SZ patients [27]. Therefore, this study provides a new combination antipsychotic regimen of low-dose risperidone plus sertraline for the early treatment of SZ in the clinical setting. Of particular note is that the use of combination therapy in FEMN SZ patients was not based on a certain threshold of depression symptoms. Although antidepressant treatments may be risky in this population, as previous studies have reported that antidepressant use is associated with an increased risk of suicide in some patients (suicidal ideation cannot be ignored as an adverse effect) [28, 29], in our study, no suicidal ideation was reported in FEMN SZ patients after 24 weeks of sertraline use. We speculate that this may be due to the lower than usual dose of sertraline taken by the patients in this study..
We hypothesize that the better efficacy of the combination of antipsychotics for SZ is due to the modulatory effect of sertraline on the 5-HT system. The effect of risperidone on the brain is mainly to reduce the activity of the dopaminergic pathway, thus reducing psychotic symptoms [30]. Negative symptoms and depressive symptoms have been reported to be associated with the dysfunction of DA and 5-HT neurons. However, the effect of risperidone on the 5-HT receptors in the brain was minimal. We found that the combination was efficacious in negative and depressive symptoms in SZ patients, which may be due to the selective inhibition of 5-HT reuptake by sertraline. Indeed, sertraline could improve the reduction of depressive symptoms by enhancing the activity of central dopaminergic neurons through the inhibition of DA transporters and reuptake [31, 32]. Moreover, the fact that low-dose risperidone combined with sertraline is more effective in reducing positive symptoms of SZ may also be due to the impact of sertraline. It may be that sertraline significantly reduced depression and negative symptoms by reducing positive symptoms of SZ. Risperidone, which targets DA activity, significantly improves positive symptoms closely related to DA dysfunction in the brain striatal, while increased 5-HT levels of sertraline may block DA activity and further improve positive symptoms of SZ [33, 34]. In addition, previous studies support that sertraline in combination with antipsychotics may reduce positive symptoms in SZ patients [35, 36]. Thus, the pharmacological effects of sertraline may explain the advantages of this promising combination treatment strategy for patients with SZ.
Notably, we found lower adverse effects in the RS group, including prolactin levels and EPS symptoms than with risperidone monotherapy. Evidence supports that high dose of atypical antipsychotics (e.g. olanzapine or risperidone) causes adverse effects in Parkinson’s disease at rates similar to those of low potency typical antipsychotics (chlorpromazine) [37]. Our findings provide further evidence that low-dose of antipsychotic combinations do not lead to an increased overall risk of antipsychotic-related adverse reactions. Previous studies have shown that risperidone use is associated with a dose-dependent elevation of prolactin in SZ patients. For example, a study of young men treated with risperidone showed that 68% of patients with SZ had prolactin levels above the upper limit of normal and that the effect of risperidone on prolactin was dose-dependent [38]. In another study of adolescent patients with autism, prolactin levels were found to be lower in the low-dose risperidone group than in the high-dose risperidone group [39]. Our large study of 198 patients with FEMN showed that a low dose of risperidone in the RS group significantly alleviated the rapid increase in prolactin levels and reduced EPS symptoms compared to a high dose in the control group.
This study also demonstrated that risperidone combined with sertraline significantly improved psychosocial functioning in FEMN patients with SZ. In addition, improvements in psychotic symptoms, depressive symptoms, and changes in serum prolactin levels were associated with improvements in psychosocial functioning. Patients with SZ usually show deficits in various psychosocial domains, such as the quality of social relationships, self-care abilities, and occupational domains [40,41,42,43]. As a result, they are at risk of unemployment, less likely to engage in social activities, and usually have no or few satisfying intimate relationships [44]. Our study is consistent with previous studies, showing that improvements in psychosocial functioning are associated with improvements in clinical symptoms and adverse reactions in SZ patients [45, 46]. Notably, a previous study by our group showed that low-dose ziprasidone combined with sertraline was efficacious for psychotic symptoms and psychosocial functioning in FEMN SZ, with fewer adverse effects compared to standardized doses of ziprasidone [47], suggesting that the combination therapy approach is also applicable to other types of antipsychotics and that the use of a low-dose antipsychotics in combination is a better option in patients with SZ.
In the present study, we noted some limitations. The first limitation was that this was not completely a double-blind placebo-controlled study. Although the nurses did not tell the patients what medications they were taking and the patients did not mutually know the number of capsules used, the patients may have identified the intervention because the RS group took two capsules and the control group took one capsule. Second, a study design comprises 4 groups, including regular dose risperidone monotherapy, low-dose risperidone monotherapy, regular dose risperidone plus sertraline, and low-dose risperidone plus sertraline will be better to answer if sertraline can have additional benefits, either with a better effect on improved psychotic/affective symptoms or lower adverse effects. Third, this study was a 24 week clinical trial, which is too short to draw firm conclusions. In particular, in this study, sertraline was administrated during psychotic onset and not throughout the whole treatment of SZ, which is a chronic disorder that usually requires long-term treatment with antipsychotics. Therefore, since the investigation was conducted solely during the first 24 weeks of treatment, there should not be any generalizations beyond this period. Fourth, another limitation was to extrapolate the results obtained on every other antipsychotic medication, as they can significantly differ in their mechanisms of action. Fifth, the study population was limited to Asians, which may limit the generalization of the findings in this study to other populations.
In conclusion, the present study suggests that low-dose risperidone combined with sertraline has a better therapeutic effect on psychotic symptoms and social functioning in FEMN SZ patients than standard doses of risperidone alone. Our study is the first to report that sertraline combined with low-dose risperidone is superior to conventional therapeutic doses of risperidone, providing a new and clinically meaningful contribution to the treatment of the early stages of SZ. These findings are promising, however, further multicenter studies of antipsychotic drug combinations in patients with FEMN SZ are warranted to confirm the preliminary findings.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- SZ:
-
Schizophrenia
- FEMN:
-
First-episode and medication-naïve
- RS group:
-
Low-dose risperidone in combination with sertraline
- PANSS:
-
Positive and negative syndrome scale
- HAMD:
-
Hamilton depression rating scale
- PSP:
-
Personal and social performance scale
- CATIE:
-
The national institute of mental health initiated the clinical antipsychotic trials of intervention effectiveness
- SSRI:
-
Selective 5-hydroxytryptamine reuptake inhibitor
- 5-HT:
-
Serotonin
- DSM-IV:
-
Diagnostic and statistical manual of mental disorders-IV
- ECG:
-
Electrocardiogram
- ESRS:
-
Extrapyramidal symptom rating scale
- ANOVA:
-
One-way analysis of variance
- RM-MANOVA:
-
Repeated measures multivariate analysis of variance
References
Barnett R. Schizophrenia. Lancet. 2018;391:648.
Poon AWC, Curtis J, Ward P, Loneragan C, Lappin J. Physical and psychological health of carers of young people with first episode psychosis. Australas Psychiatry. 2018;26:184–8.
Cheng Z, Yuan Y, Han X, Yang L, Cai S, Yang F, et al. An open-label randomised comparison of aripiprazole, olanzapine and risperidone for the acute treatment of first-episode schizophrenia: eight-week outcomes. J Psychopharmacol. 2019;33:1227–36.
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216–29.
Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56.
Galderisi S, Kaiser S, Bitter I, Nordentoft M, Mucci A, Sabé M, et al. EPA guidance on the treatment of negative symptoms in schizophrenia. Eur Psychiatry. 2021;64:e21.
Himei A, Okamura T. Evaluation of the clinical efficacy of risperidone for untreated and treated cases of schizophrenia from various aspects. Psychiatry Clin Neurosci. 2005;59:556–62.
Hunter RH, Joy CB, Kennedy E, Gilbody SM, Song F. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev. 2003. https://doi.org/10.1002/14651858.CD000440.
Williams R. Optimal dosing with risperidone: updated recommendations. J Clin Psychiatry. 2001;62:282–9.
Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346:16–22.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51.
Vázquez-Bourgon J, Mayoral-van Son J, Gómez-Revuelta M, Juncal-Ruiz M, Ortiz-García de la Foz V, Tordesillas-Gutiérrez D, et al. Treatment discontinuation impact on long-term (10-Year) weight gain and lipid metabolism in first-episode psychosis: results from the PAFIP-10 cohort. Int J Neuropsychopharmacol. 2021;24:1–7.
Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18:208–24.
Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64–77.
Zhand N, Labelle A, Ghanem D, Gujral P, Han T, Huneault G, et al. Comparison of extrapyramidal symptoms among outpatients with schizophrenia on long-acting injectable antipsychotics. J Clin Psychopharmacol. 2022;42:475–9.
Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370.
Miller DD, Caroff SN, Davis SM, Rosenheck RA, McEvoy JP, Saltz BL, et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry. 2008;193:279–88.
Doogan DP, Caillard V. Sertraline: a new antidepressant. J Clin Psychiatry. 1988;49(Suppl):46–51.
Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharmacol. 2010;647:90–6.
Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41.
Olvey EL, Skrepnek GH. The cost-effectiveness of sertraline in the treatment of depression. Expert Opin Pharmacother. 2008;9:2497–508.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–9.
Guy W. (1976) Clinical global impression. In: ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare. 217–222.
Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9.
Mulholland C, Lynch G, King DJ, Cooper SJ. A double-blind, placebo-controlled trial of sertraline for depressive symptoms in patients with stable, chronic schizophrenia. J Psychopharmacol. 2003;17:107–12.
Akiskal HS, Benazzi F, Perugi G, Rihmer Z. Agitated “unipolar” depression re-conceptualized as a depressive mixed state: implications for the antidepressant-suicide controversy. J Affect Disord. 2005;85:245–58.
Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Agitated “unipolar” major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry. 2006;67:712–9.
Marsden CA. Dopamine: the rewarding years. Br J Pharmacol. 2006;147(Suppl 1):S136–44.
Popli AP, Fuller MA, Jaskiw GE. Sertraline and psychotic symptoms: a case series. Ann Clin Psychiatry. 1997;9:15–7.
Richelson E. Pharmacology of antidepressants–characteristics of the ideal drug. Mayo Clin Proc. 1994;69:1069–81.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9.
Thakore JH, Berti C, Dinan TG. An open trial of adjunctive sertraline in the treatment of chronic schizophrenia. Acta Psychiatr Scand. 1996;94:194–7.
Opoka SM, Lincoln TM. The effect of cognitive behavioral interventions on depression and anxiety symptoms in patients with schizophrenia spectrum disorders: a systematic review. Psychiatr Clin North Am. 2017;40:641–59.
Terevnikov V, Joffe G, Stenberg JH. Randomized controlled trials of add-on antidepressants in schizophrenia. Int J Neuropsychopharmacol. 2015. https://doi.org/10.1093/ijnp/pyv049.
Rochon PA, Stukel TA, Sykora K, Gill S, Garfinkel S, Anderson GM, et al. Atypical antipsychotics and parkinsonism. Arch Intern Med. 2005;165:1882–8.
Stevens JR, Kymissis PI, Baker AJ. Elevated prolactin levels in male youths treated with risperidone and quetiapine. J Child Adolesc Psychopharmacol. 2005;15:893–900.
Kent JM, Kushner S, Ning X, Karcher K, Ness S, Aman M, et al. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J Autism Dev Disord. 2013;43:1773–83.
Vita A, Barlati S. Recovery from schizophrenia: is it possible? Curr Opin Psychiatry. 2018;31:246–55.
Kossmann C, Heller J, Brüne M, Schulz C, Heinze M, Cordes J, et al. Assessment of psychosocial functioning in a large cohort of patients with schizophrenia. Psychiatr Q. 2021;92:177–91.
Schaub D, Brüne M, Jaspen E, Pajonk FG, Bierhoff HW, Juckel G. The illness and everyday living: close interplay of psychopathological syndromes and psychosocial functioning in chronic schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2011;261:85–93.
Suttajit S, Arunpongpaisal S, Srisurapanont M, Thavichachart N, Kongsakon R, Chantakarn S, et al. Psychosocial functioning in schizophrenia: are some symptoms or demographic characteristics predictors across the functioning domains? Neuropsychiatr Dis Treat. 2015;11:2471–7.
Hakulinen C, McGrath JJ, Timmerman A, Skipper N, Mortensen PB, Pedersen CB, et al. The association between early-onset schizophrenia with employment, income, education, and cohabitation status: nationwide study with 35 years of follow-up. Soc Psychiatry Psychiatr Epidemiol. 2019;54:1343–51.
Best MW, Law H, Pyle M, Morrison AP. Relationships between psychiatric symptoms, functioning and personal recovery in psychosis. Schizophr Res. 2020;223:112–8.
Neumann E, Rixe J, Haussleiter IS, Macdonald L, Rabeneck E, Bender S, et al. Psychosocial functioning as a personal resource promoting a milder course of schizophrenia. J Psychiatr Res. 2022;148:121–6.
Zhu C, Guan X, Wang Y, Liu J, Kosten TR, Xiu M, et al. Low-dose ziprasidone in combination with sertraline for first-episode drug-naïve patients with schizophrenia: a randomized controlled trial. Neurotherapeutics. 2022;19:1037–46.
Acknowledgements
None.
Funding
Funding for this study was provided by A Nation Funded Project for Regional Science and Technology Development (Basic Research) Project (No. YDZJSX2022A064), Key Research Project in Shanxi Province (201803D31098), Special Grant 136 of First Hospital of Shangxi Medical University Project (Y2022136006) and Grant for clinical research by Wu Jieping Medical Foundation (320.6750.18336), the Science and Technology Program of Guangzhou (202206060005, 202201010093, SL2022A03J01489, 2023A03J0856, 2023A03J0839), Guangdong Basic and Applied Basic Research Foundation Outstanding Youth Project (2021B1515020064), and Medical Science and Technology Research Foundation of Guangdong (A2023224). The funding for this study had no further role in study design, data analysis, and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
XL, FW and XYZ were responsible for study design, statistical analysis, and manuscript preparation. XL, MX, and XZ were responsible for recruiting the patients, performing the clinical rating, and collecting the clinical data. XL and XZ were evolving the ideas and editing the manuscript. XZ and FW were involved in writing the protocol and co-wrote the paper. All authors have contributed to and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Ethics Committee of First Hospital of Shanxi Medical University. The work on patients was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Consent for publication
All authors have read and approved the content and agree to submit it for consideration for publication in the journal.
Competing interests
No competing interests was disclosed for each author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure 1. Flow diagram of included studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lang, X., Xue, M., Zang, X. et al. Efficacy of low-dose risperidone in combination with sertraline in first-episode drug-naïve patients with schizophrenia: a randomized controlled open-label study. J Transl Med 21, 432 (2023). https://doi.org/10.1186/s12967-023-04272-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04272-7