Abstract
Rationale
The administration of glucocorticoids (GC) as an adjunct to exposure represents a promising strategy to improve one-session exposure outcome in anxiety disorders. It remains to be determined whether similar effects can be induced with the use of acute stress. Furthermore, the possible modulation of exposure effects by hormonal factors (e.g., use of oral contraceptives (OCs)) was not explored so far.
Objectives
We investigated whether acute stress prior to one-session exposure for spider fear affects its efficacy in women using oral contraceptives (OC) relative to free-cycling (FC) women. In addition, effects of stress on generalization of exposure therapy effects towards untreated stimuli were examined.
Methods
Women with fears of spiders and cockroaches were randomly assigned to a Stress (n = 24) or No-Stress (n = 24) condition prior to one-session exposure. Of these 48 participants, 19 women used OC (n = 9 in the Stress, and n = 10 in the No-Stress group). All FC women had a regular menstrual cycle and were tested only in the follicular phase of their menstrual cycle. Pre-exposure stress induction was realized with the socially evaluated cold-pressor test. Exposure-induced changes towards treated and untreated fear stimuli were tested with behavioral approach tests for spiders and cockroaches and subjective fear and self-report measures.
Results
Acute stress did not influence exposure-induced reduction in fear and avoidance of the treated stimuli (spiders). Similarly, stress had no effect on the generalization of exposure-therapy effects towards untreated stimuli (cockroaches). Exposure-induced reduction in subjective fear and self-report measures for treated stimuli was less evident in women using OC specifically after pre-exposure stress. Women using OC had higher levels of subjective fear and scored higher in self-report measures at post-treatment (24 h after exposure) and follow-up (4 weeks after exposure).
Conclusions
OC intake may represent an important confounding factor in augmentation studies using stress or GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Specific fears are highly prevalent in the general population (cf. Wardenaar et al. 2017). Prevalence (Stinson et al. 2007) and incidence rates (Angst et al. 2016) are higher in females as compared to males (Kessler et al. 2005; McLean et al. 2011; Regier et al. 1990). Individuals with specific fears (e.g. fear of spiders) often also display fears to different stimuli from the same category (e.g. cockroaches) (Davey 1991; Matchett and Davey 1991). Therefore, generalization of fear responses to stimuli from the same or other categories seems to be a common phenomenon (Preusser et al. 2017; Waters et al. 2021).
Exposure therapy is an effective therapeutic intervention to reduce fear and avoidance in patients with specific fears (Hoffman and Smits 2008). The beneficial effects of exposure might be restricted to the specific stimulus used during the exposure therapy, which is suggestive of a reduced generalization of exposure-based fear reduction across different fear stimuli (Rowe and Craske 1998a, 1998b). Systematic research on the generalization of exposure across different fear stimuli has been initiated only recently (Zlomuzica et al. 2020; Preusser et al. 2017).
Behavioral interventions and pharmacological manipulations have been proposed to promote extinction learning and exposure therapy outcome (Craske et al. 2022). The administration of glucocorticoids (GCs) can potentiate the efficacy of exposure therapy, both in terms of the extent of symptom relief and shortening of the duration of therapeutic interventions (De Quervain et al. 2017, 2011; Soravia et al. 2006, 2014). The facilitating effects of glucocorticoids, however, critically depend on the frequency and timing of glucocorticoid administration (Raeder et al. 2019b; De Quervain et al. 2019).
Stress might equally promote exposure therapy outcome (Meir Drexler et al. 2018, 2019) albeit this has not been investigated so far. It is well known that acute stress activates the hypothalamus-pituitary-adrenocortical axis. Corticotropin releasing hormone secretion from the hypothalamus stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH) into the blood circulation. In a final step, ACTH stimulates the adrenal glands to release glucocorticoids such as cortisol (Herman et al. 2016).
Pre-extinction stress can promote subsequent fear extinction learning and extinction retrieval (Meir Drexler et al. 2017), probably also in a sex-dependent manner (Bentz et al. 2013; Meir Drexler et al. 2019; Merz and Wolf 2017; Merz et al. 2018). Oral contraceptive (OC) use and naturally occurring changes in estrogens and progesterone can influence fear extinction (Graham and Milad 2013; Merz et al. 2018; Glover et al. 2015; Maeng and Milad 2015; Stockhorst and Antov 2016). Reduced estradiol levels in women using OCs (OC) relative to estradiol levels in free-cycling (FC) women in the early follicular phase of the menstrual cycle may account for impaired fear extinction processes (Graham and Milad 2013; Merz et al. 2018). The influence of sex, OC use and variations in estrogen levels during the menstrual cycle in the context of exposure treatment, however, has been largely neglected (but see Graham et al. 2018; Raeder et al. 2019a; Zlomuzica et al. 2020). There is some evidence for a reduced symptom relief following exposure therapy in women using OCs (Graham et al. 2018; Raeder et al. 2019a). Thus, although stress-induced elevations of cortisol concentrations prior to exposure might be suitable to promote exposure therapy efficacy, such effects on exposure therapy might be further modulated by OC intake. In addition, possible changes in stress-response in women using OC need to be considered (Jentsch et al. 2022; Kirschbaum and Hellhammer 1994; Kirschbaum et al. 1996).
In the present study, we recruited women with multiple fears (spiders and cockroaches) and asked whether pre-exposure stress induction via socially evaluated cold-pressor test (SECPT; Schwabe et al. 2008) would facilitate the exposure therapy effects. We further asked whether the beneficial effects of spider exposure would generalize to other fear stimuli (cockroaches; adapted from Preusser et al. 2017). Additionally, we investigated whether OC use in women (showing reduced estradiol levels due to OC intake), in contrast to FC women in the early follicular phase of the menstrual cycle (showing low estradiol levels; see Graham and Milad 2013) would influence the effect of stress on exposure efficacy and generalization. Salivary cortisol levels in healthy controls usually begin to rise in the night and have a peak in the morning after awakening, then decline over the course of the day. Exposure therapy in the morning, when cortisol levels are high, seems to be more effective in posttreatment and follow-up assessments, as compared to exposure in the evening (Lass-Hennemann and Michael 2014). To account for such time of day effect in the present study, stress was applied in the afternoon when cortisol levels are low.
Methods
Participants
The study was preregistered on ClinicalTrials.gov (Identifier: NCT03505437). Female participants with a fear of spiders and cockroaches were recruited via various advertisements (e.g., social media groups and online bulletin boards). Participants with a history of a mental disorder, other than a specific fear for spiders and cockroaches, were not invited to the study. Other exclusion criteria involved the presence of neurological and/or endocrine disorders, pregnancy, current pharmacological treatment, and current smoking (> than five cigarettes per month). Finally, participants with a body mass index (BMI) greater than 27 or smaller than 19 kg/m2 and shift-workers were excluded.
A total of 104 individuals took part in a pre-experimental telephone screening to check for inclusion/exclusion criteria. Participants were not required to fulfill the diagnosis of a specific phobia although they had to present a substantial degree of fear towards spiders and/or cockroaches. The latter was ascertained on the basis of specific self-report measures, i.e. the Fear of spiders questionnaire (FSQ; Rinck et al. 2002) and the Fear of cockroaches questionnaire (FCQ; Scandola et al. 2010). After this screening, 77 individuals remained eligible for participation. A total of 29 individuals dropped out of the study for one of the following reasons: No longer interested in participation (n = 13), unable to attend (n = 4) or not showing up for their appointment (n = 12).
The final sample, therefore, included 48 women who were assigned to either the stress or the No-Stress group. In particular, 15 FC and 9 OC women were included to the No-Stress groups, while 14 FC and 10 OC women were included in the Stress group. All FC women had a regular menstrual cycle and were tested only in the follicular phase of their menstrual cycle (i.e., between the 3rd and 9th day after menstruation onset). FC and OC status as well as the exact phase of the menstrual cycle were determined via self-reports using a standardized questionnaire. OC women were tested during their OC intake phase. Participants were instructed to abstain from eating, drinking, or smoking and not to engage in any kind of excessive exercise for approximately 90 min prior to each of the three experimental sessions. The exclusion criteria and abstinence instructions have been selected in accordance with the pertinent literature in order to reduce confounding factors that have been shown to affect acute stress-induced salivary cortisol concentrations (De Punder et al. 2019; Kirschbaum et al. 1993, 1995, 1996; Kudielka et al. 2004, 2009).
All experimental procedures were approved by the ethics committee of the Faculty of Psychology of the Ruhr University Bochum and carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent and received a compensation of 60€ for the completion of the study.
Measures
Anxiety and depression questionnaires
Possible differences in anxiety and depression levels between the stress and No-Stress group were assessed with the Beck depression inventory-II (BDI-II; Hautzinger et al. 2006) and the State-trait anxiety inventory (STAI-S and STAI-T; Laux 1981). The BDI-II is a 21-item questionnaire used for assessing self-rated severity of depression in both clinical and nonclinical samples. The STAI consists of 40 items for measuring two types of anxiety, i.e. state anxiety and trait anxiety.
Spider and cockroach fear-related questionnaires
Different aspects related to the fear of spiders and cockroaches were assessed by using a battery of questionnaires. We used (1) the German version of the FSQ (Rinck et al. 2002), which includes 18 items that are scored on a 7-point Likert scale, (2) the Spider phobia questionnaire (SPQ; Hamm 2006) which comprises 31 dichotomously coded (i.e., true vs. false) items, and (3) the Spider beliefs questionnaire (SBQ; Pössel and Hautzinger 2003) which measures dysfunctional beliefs on 48 items according to a scale from 0 (“not at all”) to 100 (“completely”). For fear of cockroaches, the FCQ (Scandola et al. 2010), which is an exact adaptation of the FSQ but only relating to cockroaches, and a modified version of the SBQ to reflect beliefs about cockroaches (see Botella et al. 2010) were applied. Higher scores reflect a higher level of fear in each of the questionnaires.
Behavioral approach test (BAT)
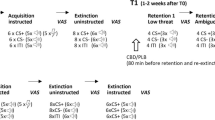
Both fear and avoidance of spiders and cockroaches were assessed with separate and independent BATs at the beginning of the first experimental session (“Pre”), at the post-stress-treatment and guided spider exposure efficacy evaluation stage (“Post”), as well as during the long term fear reduction stability assessment and generalization stage (“Follow-up”; Fig. 1; adapted from Preusser et al. 2017). To this end, the fearful stimulus (spiders: Tegenaria domestica, 1 cm; cockroaches: Blaptica dubia, 4 cm) was placed in a transparent plastic container at the far end of a 3 m x 3 m room. Participants were instructed to perform different steps with increasing difficulty. The steps involved entering the room, approaching the fearful stimulus as fast as possible, removing it from the jar of container one and ultimately placing it into the jar of container two, which was located at a distance of 1.5 m to container one. On each step, participants were instructed to only continue their approach behavior if they experienced that the fear is tolerable. When the participants indicated that fear is intolerable for continuation, the BAT was stopped.
Outline of the experimental design and main outcome measures. At the beginning of the experiment the participants underwent two BATs (spider and cockroach), and thereafter, presented with spider and cockroach-related questionnaires (Pre-SECPT treatment stage). Thereafter, the participants underwent the SECPT either under the stress condition with hands submerged in cold water or under the no-stress condition with hands submerged in warm water. Twenty-five minutes after the SECPT, all participants received a short psychoeducation and in-vivo guided spider exposure. After a delay of 24 h, the spider BAT was repeated and both the spider and the cockroach fear-related questionnaires were presented (Post-treatment phase). Long-term effects of exposure were evaluated four weeks later (Follow-up phase). At follow-up, both spider and cockroach BATs were repeated and spider and the cockroach fear-related questionnaires were presented. Abbreviations: BAT: Behavioral avoidance test, SECPT: Socially evaluated cold-pressor test
Both the spider and cockroach BATs were scaled in approach distance which comprised fifteen different steps according to a modified and expanded version of the BAT introduced by Lass-Hennemann and Michael (2014). The steps ranged from 0 (= refusal to enter the test room) to steps 1–13 (entering the room and approaching the terrarium placed at the corner of the room, touching the terrarium, placing the hand inside the terrarium etc.) and the final step 14 (= successfully placing the stimulus into a target jar that was positioned in another container).
Subjective units of distress scale (SUDS)
The participant’s subjective fear at the initial and final approach distance during the BAT was recorded (Raeder et al. 2019a, b). The SUDS with scores ranging from 0 (= no fear) to 100 (= excessive fear) served as the primary measure of subjective fear (reported verbally) during the BAT and the exposure session.
Socially evaluated cold-pressor test (SECPT)
Participants were exposed to either the stress or the control condition of the SECPT (adapted from Schwabe et al. 2008). Participants in the Stress group were instructed to place their dominant hand, including their forearm, into a small tub which contained ice water with a temperature between 0 °C and 3 °C for a duration of three minutes. An additional experimenter was present, observing and videotaping the participant during the procedure. If participants were not able to tolerate the cold water for the duration of three minutes, they were instructed to place their arm above the water in the repository. Participants in the control group had to place their dominant hand into warm water with a temperature of 36 °C to 37 °C. To assess activation of the sympathetic nervous system, blood pressure (i.e., systolic and diastolic (mmHg)) was assessed at nine times in total. These referred to three times before (baseline), during (peak), and after (post) the stress induction or no-stress control procedure. Mean values were computed for each assessment time. After the procedure, participants had to indicate on a scale from 0 (i.e., “not at all”) to 100 (i.e., “very much”) how difficult, unpleasant, stressful, and painful the experience was felt (ratings adapted from Schwabe et al. 2008).
Salivary cortisol
Salivary cortisol concentrations were measured as a marker for HPA axis activity (Kirschbaum and Hellhammer 1994). Samples were taken with saliva sampling tubes (Sarstedt, Nümbrecht, Germany) on different time points across the course of the experiment (please refer to Table 1 for a precise overview). On Day 1, which included the pre-treatment screening, SECPT and exposure session, samples 1–7 were taken. On Day 2, which included the post-treatment BAT for spiders another two samples (8–9) were taken. On Day 3, which included the follow-up assessment with BATs for spiders and cockroaches, another two samples (10–11) were taken. Chemiluminescence immunoassay with high sensitivity (IBL International, Hamburg, Germany) was used to measure salivary cortisol concentrations. The intra- and inter-assay coefficients of variance for cortisol were both below 8.0%.
One-session exposure treatment
The exposure session consisted of the first eight steps of a standardized fourteen-step fear hierarchy progression procedure (steps increased in difficulty; see Mystkowski et al. 2006 for details). The experimenter assessed individual levels of fear (SUDs) by questioning the participant during the performance of each step. Each step needed to be performed until the participant reached a SUDs rating of 30 or lower. The exposure session was terminated after 45 min or once the participant had completed all steps.
Experimental design and procedure
The study comprised three experimental phases which were conducted on three separate days (see Fig. 1), all of which were conducted in the afternoon (1 to 5 p.m.) to reduce daytime-related variability in stress hormone secretion. The first phase on Day 1 started with two BATs (first spider BAT, then cockroach BAT) and the completion of the BDI-II, STAI, as well as the spider and cockroach-fear related questionnaires (i.e., SPQ, FSQ/FCQ, SBQ/CBQ). After that, participants had to complete either the cold water (Stress group) or the warm water (No-Stress group) condition of the SECPT. Thereafter, participants received a brief psychoeducation session about specific phobia and subsequently engaged in the in-vivo guided spider exposure, which commenced 25 min after the participants had immersed their hand into the water. The entire first phase on Day 1 lasted approximately 140 min. The second phase on Day 2 (of approximately 45 min duration) took place 24 h later after the first phase and involved a post-treatment measurement consisting of spider BAT and both the spider and the cockroach fear-related questionnaires. The third phase (of approximately 45 min duration) took place four weeks later and involved a follow-up assessment. Here, both BATs as well as the completion of the spider and the cockroach fear-related questionnaires were conducted. At the end of the third phase, participants were debriefed and received their compensation.
Statistical analyses
Statistical analyses were performed with SPSS Statistics for Macintosh, Version 27.0 (Armonk, NY: IBM Corp.). Pre-exposure participant characteristics and stress ratings were compared using a series of two-way ANOVAs with the between-subjects factors “Stress” (Stress vs. No-Stress) and “OC” (OC vs. FC). Blood pressure as well as salivary cortisol were analyzed with ANOVAs with the within-subjects factor “Time” (salivary cortisol: baseline vs. 25 min after SECPT; blood pressure: measurements at baseline, during stress induction vs. after the SECPT) and the between-subjects factors “Stress” and “OC”. Exposure-induced changes in outcome measures (i.e., BAT score, subjective fear during the BAT, questionnaires) were also analyzed with two-way ANOVAs with three levels of the within-subjects factor “Time” (Day 1 Pre, Day 2 Post, vs. Day 3 Follow-up). Note that analyses of the cockroach BAT score and subjective fear at the initial and final approach distances comprised only two levels (Pre vs. Follow-up). ANOVA results were considered significant when P-values smaller than 0.05 were found. Bonferroni-corrected multiple pair-wise group comparisons were made with T-tests for independent samples and considered to be significant when p-values smaller than P = 0.016 emerged.
Results
Pre-exposure participant characteristics
As displayed in Table 2, all experimental groups were comparable with respect to age (Main effect of OC: F(1,44) = 1.659; P = 0.204; Main effect of Stress: F(1,44) = 0.280; P = 0.599; Interaction: F(1,44) = 0.491; P = 0.487; two-way ANOVA; Table 2), BMI (Main effect of OC: F(1,44) = 1.129; P = 0.294; Main effect of Stress: F(1,44) = 0.0001; P = 0.991; Interaction: F(1,44) = 0.735; P = 0.396), and their scores on the BDI-II (Main effect of OC: F(1,43) = 0.771; P = 0.385; Main effect of Stress: F(1,43) = 1.369; P = 0.248; Interaction: F(1,43) = 0.981; P = 0.327), and the STAI-T before exposure (Main effect of OC: F(1,44) = 0.262; P = 0.611; Main effect of Stress: F(1,44) = 0.323; P = 0.573; Interaction: F(1,44) = 1.987; P = 0.166). We additionally compared FC and OC groups with respect to demographical data, salivary cortisol, blood pressure and stress ratings separately for No-Stress and Stress conditions. No significant differences between FC and OC groups were found for these comparisons (all Ps > 0.1; see Table 3 for a listing of comparisons and corresponding P-values).
Most importantly, there were no pre-treatment group differences in the spider- and cockroach-fear related questionnaires (FSQ: Main effect of OC: F(1,44) = 1.228; P = 0.274; Main effect of Stress: F(1,44) = 2.331; P = 0.134; Interaction: F(1,44) = 1.023; P = 0.317, FCQ: Main effect of OC: F(1,44) = 1.770; P = 0.190; Main effect of Stress: F(1,44) = 0.298; P = 0.588; Interaction: F(1,44) = 0.470; P = 0.496), as well as in subjective fear at the initial approach distance to the spider and the cockroach during the pre-treatment BAT (Spider SUDS: Main effect of OC: F(1,44) = 0.210; P = 0.886; Main effect of Stress: F(1,44) = 0.446; P = 0.508; Interaction: F(1,44) = 0.309; P = 0.581, Cockroach SUDS: Main effect of OC: F(1,44) = 1.067; P = 0.307; Main effect of Stress: F(1,44) = 0.187; P = 0.668; Interaction: F(1,44) = 0.054; P = 0.817).
The analysis of approach behavior during the pre-treatment BAT yielded a significant main effect of OC (Spider approach: Main effect of OC: F(1,44) = 12.010; P = 0.001, Cockroach approach: Main effect of OC: F(1,44) = 12.758; P = 0.001). Women who used OC showed less approach behavior during the pre-treatment BAT with the spider (M = 4.79, SD = 1.40) and the cockroach (M = 5.00, SD = 1.92) as compared to the FC group (Spider: M = 6.69, SD = 2.04; Cockroach: M = 7.17, SD = 2.07). In contrast, no significant main effect of the grouping factor Stress (Spider approach: Main effect of Stress: F(1,44) = 0.406; P = 0.527, Cockroach Approach Index: Main effect of Stress: F(1,44) = 0.026; P = 0.873) or a OC x Stress interaction effect was found for approach behavior during the pre-treatment BAT measurement (Spider approach: Interaction: F(1,44) = 0.084; P = 0.773, Cockroach Approach Index: Interaction: F(1,44) = 0.026; P = 0.873).
Stress-response manipulation check
Stress-effects on salivary cortisol concentrations
Two participants were excluded from data analysis, because of undetectable cortisol concentrations, resulting in a sample size of 23 participants in both the Stress and No-Stress groups. The trajectory of salivary cortisol concentrations across the measures 1–7 on experimental Day 1, measures 8–9 on experimental Day 2, and measures 10–11 on Day 3 is illustrated in Fig. 2. In order to determine whether the stress induction would raise salivary cortisol concentrations, we compared the last measurement before stress induction (sample point 4 on experimental day one) with the first measurement after stress induction (sample point 5) using a two-way ANOVA with repeated measures. As expected, stress induction significantly increased salivary cortisol concentrations as indicated by a significant interaction of the factors Time and Stress (F(1,42) = 7.573; P = 0.009; Table 2). Main effects of the factors Time, OC and Stress were not statistically significant (Time: F(1,42) = 0.636; P = 0.430, OC: F(1,42) = 0.395; P = 0.533, Stress: F(1,42) = 1.183; P = 0.283). The remaining first and second order interactions between the factors Time, OC and Stress were also all non-significant (OC vs. Stress: F(1,42) = 0.209; P = 0.650, Time x OC: F(1,42) = 0.245; P = 0.623, Time x OC x Stress: F(1,42) = 0.0000146; P = 0.990). Post-hoc group comparisons indicated that the Stress and No-Stress groups had similar cortisol concentrations at baseline (T[44] = 0.912; P = 0.367; T-test for independent samples), but 25 min after the stress induction, participants exposed to cold water (Stress group), showed significantly higher salivary cortisol concentrations as compared to the warm-water exposed participants (No-Stress group: T[28.995] = -2.059; P = 0.049; Fig. 2).
Monitoring of salivary cortisol concentrations of the Stress and No-Stress groups. Each data point represents mean and SEM salivary cortisol concentrations that have been measured during 7 sampling points on experimental day 1, two sampling points on day 2 and another two sampling points on experimental day 3 (see Table 1 for details)
Stress effects on systolic and diastolic blood pressure
Assessments of systolic and diastolic blood pressure (cf. Table 2) at baseline, during stress induction and post-stress stages also indicated a successful stress induction. Two-way ANOVA with repeated measures yielded significant effects of the factors Time and Stress as well as significant Time x Stress interactions (Systolic blood pressure: Main effect of Time, F(2,88) = 38.824; P < 0.001; Main effect of Stress, F(1,44) = 8.799, P = 0.005; Time x Stress interaction: F(2,88) = 35.381; P < 0.001, Diastolic blood pressure: Main effect of Time, F(2,86) = 54.002; P < 0.001; Main effect of Stress, F(1,43) = 6.903, P = 0.012; Time x Stress interaction: F(2,86) = 22.353; P < 0.001). The effect of stress induction on systolic and diastolic blood pressure was not affected by the OC factor. No significant main effect of the factor OC, Time x OC interaction, or OC x Stress interaction was found (Systolic blood pressure: Main effect of OC, F(1,44) = 1.839, P = 0.182; Time x OC interaction: F(2,88) = 0.437; P = 0.647; OC x Stress interaction: F(1,44) = 0.001; P = 0.976, Diastolic blood pressure: Main effect of OC, F(1,43) = 3.025, P = 0.089; Time x OC interaction: F(2,86) = 0.741; P = 0.480; OC x Stress interaction: F(1,43) = 0.356; P = 0.554).
Post-hoc analyses indicated that Stress and No-Stress groups showed similar systolic and diastolic blood pressure during the baseline measurement prior to stress induction (Systolic blood pressure: T[46] = -0.547; P = 0.587, Diastolic blood pressure: T[45] = -0.941; P = 0.352). As expected the Stress group had significantly higher systolic and diastolic blood pressure during the stress induction as compared to the No-Stress group (Systolic blood pressure: T[46] = -5.124; P < 0.001, Diastolic blood pressure: T[45] = -4.802; P < 0.001). The Stress group showed a significantly higher systolic, but not diastolic, blood pressure after the SECPT as compared to the No-Stress group (Systolic blood pressure: T[46] = -2.077; P = 0.043, Diastolic blood pressure: T[45] = -1.308; P = 0.197), suggesting that there was a small after-effect of stress induction, that, however, as indicated by the diastolic blood pressure measure, was likely in a process of rapid decline. In line with the significant effects of the stress-treatment on physiological parameters, the Stress group also experienced the SECPT as being more difficult (Main effect of Stress: F(1,43) = 283.708; P < 0.001; Table 2), unpleasant (Main effect of Stress: F(1,43) = 153.232; P < 0.001), stressful (Main effect of Stress: F(1,43) = 129.612; P < 0.001), and painful (Main effect of Stress: F(1,43) = 286.950; P < 0.001), as compared to the subjective stress ratings of the No-Stress group.
Effects of stress and OC on spider exposure efficacy: Spider BAT Behavioral approach/avoidance
The participants’ approach behavior towards spiders increased across the 3 BAT trials (Main effect of Time: F(2,88) = 97.334; P < 0.001; data not shown). The OC group showed less approach behavior as compared to the FC group as indicated by a main effect of the factor OC (F(1,44) = 13.768; P < 0.001). In contrast, approach behavior was not modulated by the factor Stress (Main effect of Stress: (F(1,44) = 0.244; P = 0.624). Furthermore, no significant OC x Stress interaction was found (F(1,44) = 0.244; P = 0.624). First and second order interactions that included the factor Time were all non-significant (Time x OC: F(2,88) = 0.260; P = 0.772, Time x Stress: F(2,88) = 0.202; P = 0.817, Time x OC x Stress: F(2,88) = 0.864; P = 0.425).
Subjective fear ratings (SUDS)
The analysis of the initial approach distance measure of the BAT suggested that the participants experienced a reduction in their subjective fear levels across the 3 trials (Main effect of Time: F(2,88) = 79.625; P < 0.001). The subjective fear reduction was modulated by both the factors OC and Stress as indicated by a significant second order interaction between Time, OC and Stress (F(2,88) = 3.135; P = 0.048). First order interactions between Time and Stress (F(2,88) = 0.820; P = 0.922; Two-way ANOVA with repeated measures), Time and OC (F(2,88) = 1.427; P = 0.246) and OC and Stress (F(2,88) = 1.357; P = 0.250), were all statistically not significant. Furthermore, the main effects of the factors OC and Stress were also not significant (OC: F(1,44) = 1.908; P = 0.174, Stress: F(1,44) = 0.328; P = 0.570).
Post-hoc analyses of the Stress group indicated that OC and FC groups experienced similar levels of subjective fear during the pre-stress session (T[21.489] = -0.303; P = 0.764; T-test for independent samples; Fig. 3A). Trends for higher levels of subjective fear in OC group were found during post-stress (T[22] = -2.420; P = 0.024), and follow-up sessions (T[22] = -2.344; P = 0.028). However, these differences failed to reach the level of statistical significance after Bonferroni-correction. No significant differences in subjective fear levels were observed in the No-Stress OC and FC groups (Pre: T[19.858] = -0.628; P = 0.537; Post: T[21.4747] = 0.167; P = 0.869; Follow-up: T[22] = -0.077; P = 0.939; Fig. 3B).
Subjective fear at the initial approach distance. A-B. Subjective fear ratings (SUDS) during the spider-BAT during pre-exposure, post-exposure, and follow-up measurements of the Stress and No-Stress groups. C.-D. Subjective fear ratings during the cockroach-BAT during pre-exposure, and follow-up measurements of the Stress and No-Stress groups. Bars represent mean and SEM of the SUDS scores. Abbreviations: FC: Free-cycling, OC: Oral contraceptive, SUDS: Subjective units of distress Scale
The subjective fear levels at the final approach distance during the pre- and follow-up BATs suggested that the participants showed a significant reduction of their subjective fear assessments (Main effect of Time: F(2,78) = 22.554; P < 0.001). No significant main effects of the factors OC and Stress were found (OC: F(1,39) = 0.000069; P = 0.993. Stress: F(1,39) = 0.305; P = 0.584). First and second order interactions were likewise statistically not significant OC x Stress: F(1,39) = 1.493; P = 0.229, Time x OC: F(2,78) = 0.474; P = 0.624, Time x Stress: F(2,78) = 1.314; P = 0.275, Time x OC x Stress: F(2,78) = 1.552; P = 0.218).
Spider fear-related questionnaires
Fear of spider questionnaire (FSQ)
Across the 3 measurements (Pre, Post, and Follow-up), participants showed a significant decrease in their fear of spider scores as indicated by a significant main effect of Time (F(2,88) = 60.783; P < 0.001; Figs. 4A,B). Furthermore, a significant mean effect of the factor OC was found, suggesting that the OC group expressed higher levels of spider fear as compared to the FC group (F(1,44) = 5.550; P = 0.023). Post-hoc analyses indicated that this effect was mainly driven by significant differences between OC and FC groups in the Stress condition. This difference was observed after the induction of stress and during the follow-up measurement, but not prior to the stress induction (Pre: T[22] = -1.834; P = 0.080; Post: T[22] = -3.374; P = 0.003; Follow-up: T[22] = -2.758; P = 0.011). No such difference between the OC and FC group was observed in the No-Stress condition (Pre: T[22] = -0.059; P = 0.954; Post: T[22] = -0.325; P = 0.748; Follow-up: T[22] = -0.453; P = 0.655). In contrast, no significant main effect of the factor Stress was found (F(1,44) = 0.726; P = 0.399). First and second order interactions between the factors Time, OC and Stress were all non-significant (Time x OC: F(2,88) = 2.178; P = 0.119, Time x Stress: F(2,88) = 0.677; P = 0.511, OC x Stress: F(1,44) = 3.331; P = 0.075, Time x OC x Stress: F(2,88) = 0.986; P = 0.377).
Spider and cockroach-related questionnaires. Pre-, post-exposure and follow-up assessment of spider- and cockroach-related questionnaires of the Stress and No-Stress groups. A.-F. Bars represent mean and SEM of the fear of spiders, spider beliefs and spider phobia scores, respectively. G.-J. Bars represent mean and SEM of the fear of cockroach, and cockroach beliefs scores, respectively. Abbreviations: FC: Free-cycling, OC: Oral contraceptive, FSQ: Fear of spiders questionnaire, SBQ: Spider beliefs questionnaire, SPQ: Spider phobia questionnaire, FCQ: Fear of cockroaches questionnaire, CBQ: Cockroach beliefs questionnaire. *: p < 0.05; T-test for independent samples
Spider beliefs questionnaire (SBQ)
Participants showed a significant decrease in their spider belief scores across the Pre, Post and Follow-up measurements (Main effect of Time: F(2,88) = 64.356; P < 0.001; Figs. 4C,D). No significant main effects of the factors OC or Stress were observed (OC: F(1,44) = 2.963; P = 0.092, Stress: F(1,44) = 0.416; P = 0.522). However, significant interactions were observed between the factors OC and Stress (F(1,44) = 4.157; P = 0.047), as well as between the factors Time x OC x Stress (F(2,88) = 3.829; P = 0.025). Similar to the fear of spiders results reported above, post-hoc analyses indicated that this effect was mainly driven by significant differences between OC and FC groups in the Stress condition. OC and FC groups showed a significant difference in their spider belief scores after the induction of stress and during the follow-up measurement, but not prior to the stress induction (Pre: T[22] = -1.494; P = 0.149; Post: T[22] = -2.920; P = 0.008; Follow-up: T[22] = -3.234; P = 0.004). Again no such difference between the OC and FC groups was observed in the No-Stress condition (Pre: T[22] = -0.198; P = 0.845; Post: T[22] = 0.398; P = 0.695; Follow-up: T[22] = 0.303; P = 0.765). Interactions between the factors Time and OC (F(2,88) = 1.315; P = 0.273) and Time and Stress (F(2,88) = 2.386; P = 0.098) were not statistically significant.
Spider phobia questionnaire (SPQ)
Similar to the fear of spider and spider beliefs results presented above, participants showed a significant decrease in their spider phobia scores across the Pre, Post, and Follow-up measurements (Main effect of Time: F(2,88) = 37.888; P < 0.001; Figs. 4E,F). Furthermore, a significant Time x OC interaction was found (F(2,88) = 3.197; P = 0.046), which was obviously mainly driven by the relatively stable spider phobia scores of the OC groups. No significant main effects of the factors OC or Stress were obtained (OC: F(1,44) = 1.310; P = 0.259, Stress: F(1,44) = 0.240; P = 0.627). Similarly, no significant interaction effects were observed (OC x Stress: F(1,44) = 0.492; P = 0.487, Time x OC: F(2,88) = -0.289; P = 0.750, Time x Stress: F(2,88) = 1.089; P = 0.341).
In sum, these results suggest OC use in combination with stress appears to reduce some of the benefits of exposure therapy on some self-report measures.
Effects of OC and Stress on the generalization of guided spider exposure effects to other fear stimuli: Cockroach BAT
Behavioral approach/avoidance
The participants’ approach behavior towards cockroaches increased from the pre-exposure measurement to the follow-up BAT measurement (Main effect of Time: F(1,44) = 54.189; P < 0.001; data not shown). The OC group showed less approach behavior as compared to the FC group, as evidenced by a main effect of the factor OC (F(1,44) = 10.595; P = 0.002).
Approach behavior towards cockroaches was not affected by the factor Stress (Main effect of Stress: (F(1,44) = 0.023; P = 0.881). First and second order interactions were all non-significant (OC x Stress: F(1,44) = 0.066; P = 0.798, Time x OC: F(1,44) = 0.124; P = 0.727, Time x Stress: F(1,44) = 0.000074; P = 0.993, Time x OC x Stress: F(1,44) = 0.488; P = 0.488). Post-hoc pairwise comparisons of the pre-exposure approach behavior of the OC and FC groups indicated that the OC groups generally showed higher avoidance of the cockroaches as compared to the FC groups (Stress condition: T[22] = 2.269; P = 0.033, No-Stress condition: T[22] = 2.838; P = 0.010). Most remarkably, the spider exposure intervention abolished this difference irrespective of the stress condition (Stress condition: T[22] = 1.765; P = 0.091, No-Stress condition: T[22] = 1.484; P = 0.152). These results suggest that the effects of spider exposure intervention indeed generalized to a different insect fear stimulus and consequently reduced avoidance behavior.
Subjective fear ratings (SUDS)
The subjective fear levels at the initial approach distance during the pre- and follow-up BATs suggested that participants experienced a significant reduction in their subjective fear levels (Main effect of Time: F(1,42) = 50.104; P < 0.001; Two-way ANOVA with repeated measures; Fig. 3C,D). No significant main effects of the factors OC and Stress were found (OC: F(1,42) = 1.341; P = 0.253. Stress: F(1,42) = 0.005; P = 0.943). First and second order interactions were likewise statistically not significant (OC x Stress: F(1,42) = 0.001; P = 0.974, Time x OC: F(1,42) = 0.016; P = 0.900, Time x Stress: F(1,42) = 0.090; P = 0.765, Time x OC x Stress: F(1,42) = 0.000039; P = 0.995).
The subjective fear levels at the final approach distance measured during the Pre- and Follow-up BATs did not show a significant decline in experienced fear level (Main effect of Time: F(1,43) = 3.249; P = 0.078). No significant main effects of the factors OC and Stress were found (OC: F(1,43) = 0.245; P = 0.623. Stress: F(1,43) = 0.327; P = 0.570). First and second order interactions were likewise statistically not significant (OC x Stress: F(1,43) = 0.057; P = 0.812, Time x OC: F(1,43) = 0.460; P = 0.501, Time x Stress: F(2,78) = 0.002; P = 0.968, Time x OC x Stress: F(1,43) = 0.288; P = 0.594).
Cockroach fear-related Questionnaires
Fear of cockroach questionnaire (FCQ)
Participants showed a significant decrease in their fear of cockroach scores across the 3 assessments (Pre, Post, and Follow-up) as indicated by a significant main effect of Time (F(2,88) = 21.525; P < 0.001; Figs. 4G,H). No significant main effects of the grouping factors OC or Stress was found (OC: F(1,44) = 3.111; P = 0.085, Stress: F(1,44) = 0.376; P = 0.543). First and second order interactions between the factors Time, OC and Stress were all non-significant (Time x OC: F(2,88) = 1.075; P = 0.346, Time x Stress: F(2,88) = 0.572; P = 0.567, OC x Stress: F(1,44) = 0.635; P = 0.430, Time x OC x Stress: F(2,88) = 0.083; P = 0.920).
Cockroach beliefs questionnaire (CBQ)
Similar to the fear of cockroach scores, participants showed a significant decrease in their cockroach belief scores across the Pre, Post and Follow-up measurements (Main effect of Time: F(2,88) = 17.437; P < 0.001; Figs. 4I,J). No significant main effects of the factors OC or Stress were observed (OC: F(1,44) = 1.110; P = 0.298, Stress: F(1,44) = 0.007; P = 0.933). First and second order interactions between the factors Time, OC and Stress were all non-significant (Time x OC: F(2,88) = 0.200; P = 0.819, Time x Stress: F(2,88) = 0.418; P = 0.660, OC x Stress: F(1,44) = 1.782; P = 0.189, Time x OC x Stress: F(2,88) = 0.125; P = 0.883).
Discussion
We have investigated the effects of acute stress on one-session exposure outcome and generalization in women with fear of spiders and cockroaches. Acute stress, administered prior to exposure, had no overall effect on exposure-induced reduction in fear and avoidance for the treated stimuli (spiders). Likewise, participants’ approach behavior towards untreated stimuli (cockroaches) increased from the pre-stress and pre-exposure measurement to the follow-up measurement, but no effect of stress induction was found. Similar findings have been obtained for subjective and self-report measures. This pattern of results suggests no effect of stress on exposure efficacy and generalization in women. However, the exposure-induced reduction in subjective fear and self-report measures for treated stimuli was reduced in women using OC specifically after pre-exposure stress induction. These results underline the importance of sex hormones as potential modulators of the efficacy and outcome of an one-session exposure treatment.
Our finding differs from previous exposure therapy augmentation studies showing that GC administration (that is associated with high cortisol levels) prior to exposure therapy leads to stronger symptom reduction as compared to a placebo condition (De Quervain et al. 2011; Soravia et al. 2006, 2014). Furthermore, it has been reported that higher physiological morning cortisol levels prior to exposure lead to stronger symptom alleviation as compared to lower evening cortisol levels prior to exposure (Lass-Hennemann and Michael 2014).
In contrast to these studies, the present results suggest that acute stress (and the accompanying physiological cortisol increase) does not promote exposure-induced reduction of fear and avoidance towards treated stimuli in women. More specifically, in FC stress did not lead to additional beneficial reduction of fear and avoidance. Women using OCs also did not benefit from acute stress, but show a less pronounced reduction in subjective fear at post-treatment and long term follow-up.
The absence of beneficial effects of pre-exposure stress on exposure efficacy and generalization was not due to a failure to induce stress. A multi-factorial manipulation check demonstrated that the Stress groups showed increased blood pressure, salivary cortisol concentrations and stress ratings after stress induction as compared to a baseline measurement immediately before stress induction. More specifically, FC women showed a slightly increased stress cortisol response. Our results also suggest that OC use does not attenuate the cortisol response to acute stress, as it was previously shown with other stressors, e.g. the Trier Social Stress Test (Gervasio et al. 2022) or bicycle ergometry (Kirschbaum et al. 1996). Thus, the modulatory effect of OC use on a one-session exposure outcome in female participants exposed to acute stress cannot be attributed to an insufficient cortisol response. However, it should be noted that the effects of laboratory stress on cortisol responses might be further dependent on the menstrual cycle phase (both in the presence or absence of OC use, see Montero-López et al. 2018). This possible confounding factor should be addressed systematically in future studies on this topic. It is noteworthy, however, that our and previous studies cannot be directly compared due to differences in exposure session protocols and sample characteristics. It has also been shown that patients with specific fears respond differently than normal volunteers to a stressor associated with social evaluation (Furlan et al. 2001). While a proportion of social phobia patients shows an increase in salivary cortisol to a social speech task, some patients show a decrease in cortisol (Furlan et al. 2001). Such a dichotomy in magnitude and in distribution of the cortisol response to a stressor might also be present in our study. This might explain why the cortisol increase in the stress group and in FC women in particular is somewhat lower than expected. Unfortunately, the small sample size in our study limits the possibility to test this conclusion. The same might be true for OC women with specific fears being exposed to stress (see Jentsch et al. 2022) who might equally show an altered pattern of cortisol responses after stress. These factors might collectively explain why the relatively low cortisol increase after stress in our study was insufficient to replicate previous findings on the beneficial effect of GCs on exposure efficacy. Stress, at least induced by the SECPT, in contrast to GC administration (De Quervain et al. 2011; Soravia et al. 2006, 2014) or daytime dependent increases in cortisol (Las-Hennemann & Michael, 2014) might be insufficient to induce cortisol levels to a point where exposure therapy effects can be further boosted. Additionally, more potent stress protocols in increasing cortisol concentrations such as the TSST should be investigated in future studies. We and others showed that OC use diminishes the exposure-induced benefit in female participants with spider phobia (Graham et al. 2018; Raeder et al. 2019a). While OC intake had no effect on the efficacy of exposure on the reduction of spider fear and avoidance under No-Stress conditions, we found a detrimental effect of OC intake on exposure efficacy under the pre-exposure Stress condition. This finding is in line with previous reports that demonstrate an antagonistic effect of OC on cortisol-induced facilitation of emotional learning in women (Merz and Wolf 2017; Merz et al. 2012). A possible explanation of this effect might be the blockade of brain estradiol synthesis by hormonal contraceptives that is exaggerated by stress. It has been shown that women taking hormonal contraceptives (causing reduced estradiol concentrations) compared to free-cycling women in the follicular phase of the menstrual cycle (showing low estradiol levels) show impairments in fear extinction memory (Graham and Milad 2013). In a study with a mixed group of men and women (Pletzer et al. 2021), social evaluative stress modulated estradiol levels in a bidirectional way. Two subgroups of responders were identified. About sixty percent of the participants showed a reduction in estradiol levels after 20 min post-stress, while the remaining 40 percent of the participants responded with an increase in estradiol levels at this stage. Most importantly, these changes were not correlated with the cortisol response to social evaluative stress (Pletzer et al. 2021). Similarly, women with low endogenous estradiol levels show a more pronounced recovery of extinguished fear measured at the level of skin conductance responses as compared to women with high estradiol levels (White and Graham 2016). Additionally, stress exposure prior to fear acquisition modulates the recall of fear extinction memories in dependence of the estradiol status of women (Antov and Stockhorst 2014; reviewed in Hsu et al. 2021). Most importantly, women with high estradiol status seem to be insensitive to declarative memory impairments induced by pre-learning stress (Antov and Stockhorst 2018). Therefore, it is conceivable that stress and OC had an additive suppressive effect on estradiol synthesis up to a point where it affected extinction learning leading to a diminution of the exposure effect on fear and avoidance reduction. Future studies should incorporate hormonal analyses to gain more insight into these mechanisms.
Another aim of this study was to investigate possible effects of stress on generalization of exposure therapy effects towards untreated stimuli (Preusser et al. 2017; Pittig et al. 2018). Research on such stimulus-based generalization of exposure therapy effects is limited (Rowe and Craske 1998a, 1998b). We previously showed that exposure therapy to alleviate spider fear and avoidance also leads to the reduction of fear and avoidance towards cockroaches (Preusser et al. 2017). This finding indicates a generalization of exposure-induced benefit across different fear categories. In the present study, we have successfully replicated this finding. Although we did not incorporate a non-treated control group, the increase in approach behavior towards cockroaches is substantial and comparable to our previous finding (Preusser et al. 2017). This suggests that exposure towards a specific fear stimulus might be helpful for coping with other fear stimuli that belong to the same fear category, for example small animal phobia in the present study.
Therapy generalization effects might be related to a generalized mastery experience or an increase in self-efficacy beliefs after exposure to the treated stimuli (Raeder et al. 2019c). Enhanced self-efficacy can promote extinction learning and retrieval (Zlomuzica et al. 2015) which in turn enhances learning experiences made during exposure therapy (Raeder et al. 2020). Whether such generalization effects can also be observed for stimuli that belong to a different fear category (Zlomuzica et al. 2020) such as heights (relative to spiders for example), remains to be explored.
Nevertheless, stress did not influence the generalization of exposure-induced benefit across different fear stimuli. Basic research on the effects of stress on stimulus-based fear extinction is very limited (but see Hagedorn et al. 2021). Findings from basic research on context-dependent generalization suggests that stress promotes generalization of extinction memories across different contexts (Meir Drexler et al. 2017) and abolishes context-dependent fear renewal (Meir Drexler et al. 2018). Acute stress might support the generalization of therapeutic effects from the treatment context to other everyday situations (Vervliet et al. 2013). The present findings, however, suggest that this conclusion does not apply for (stimulus-based) generalization of therapeutic effects from treated to untreated fear stimuli. Whether stress supports context-dependent generalization of therapy effects but not generalization of exposure-therapy effects across different stimuli remains to be explored.
Potential limitations of our study might be related to the relatively small sample size or individual differences in the amount of cortisol released following a lab stressor (van Eck et al. 1996) and other effects (i.e. experienced life stress, wake time, BMI, and caffeine intake) in this relationship. It remains to be shown whether cortisol increases or other factor are responsible for the observed effects. In order to estimate the probability of a type 2 error, that is false negative results, we performed a post-hoc power-analysis using G*Power to assess the archived power. Post-hoc power-analyses were performed on spider-BAT subjective fear ratings at the post-exposure, and follow-up stage of the Stress groups. The post-hoc power analysis yielded an achieved power (1-β error probability) of 0.9994 and 0.99811 for post-exposure and follow-up data, which points to a lower possibility of reporting false negative results. Nevertheless, in future studies, using a larger sample, it might be interesting to investigate whether the systemic administration of GC to women using OC and FC might affect the efficacy of exposure-treatment and the generalization of symptom alleviation to related and unrelated fear stimuli such as spiders, cockroaches and heights.
Conclusion
Our data highlight the complexity of the relationship between stress, hormonal status, one-session exposure outcome, and exposure-treatment generalization. Further research on the physiological, neurohormonal and cognitive mechanisms of exposure-treatment generalization is needed to obtain a more consistent picture which can be translated into innovative therapeutic approaches.
Data Availability
All data obtained during the current study are available from the corresponding author on reasonable request.
References
Angst J, Paksarian D, Cui L, Merikangas KR, Hengartner MP, Ajdacic-Gross V, Rössler W (2016) The epidemiology of common mental disorders from age 20 to 50: Results from the prospective Zurich cohort Study. Epidemiol Psychiatr Sci 25:24–32
Antov MI, Stockhorst U (2014) Stress exposure prior to fear acquisition interacts with estradiol status to alter recall of fear extinction in humans. Psychoneuroendocrinology 49:106–118
Antov MI, Stockhorst U (2018) Women with high estradiol status are protected against declarative memory impairment by pre-learning stress. Neurobiol Learn Mem 155:403–411
Bentz D, Michael T, Wilhelm FH, Hartmann FR, Kunz S, Von Rohr IRR, De Quervain DJF (2013) Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology 38:1186–1197
Botella C, Bretón-López J, Quero S, Baños R, García-Palacios A (2010) Treating cockroach phobia with augmented reality. Behav Ther 41:401–413
Craske MG, Treanor M, Zbozinek TD, Vervliet B (2022) Optimizing exposure therapy with an inhibitory retrieval approach and the OptEx Nexus. Behav Res Ther 152:104069
Davey GC (1991) Characteristics of individuals with fear of spiders. Anxiety Res 4:299–314
De Punder K, Heim C, Entringer S (2019) Association between chronotype and body mass index: The role of C-reactive protein and the cortisol response to stress. Psychoneuroendocrinology 109:104388
De Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH (2011) Glucocorticoids enhance extinction-based psychotherapy. Proc Natl Acad Sci U S A 108:6621–6625
De Quervain D, Schwabe L, Roozendaal B (2017) Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 18:7–19
De Quervain D, Wolf OT, Roozendaal B (2019) Glucocorticoid-induced enhancement of extinction-from animal models to clinical trials. Psychopharmacology 236:183–199
Furlan PM, DeMartinis N, Schweizer E, Rickels K, Lucki I (2001) Abnormal salivary cortisol levels in social phobic patients in response to acute psychological but not physical stress. Biol Psychiatry 50:254–259
Gervasio J, Zheng S, Skrotzki C, Pachete A (2022) The effect of oral contraceptive use on cortisol reactivity to the Trier Social Stress Test: A meta-analysis. Psychoneuroendocrinology 136:105626
Glover EM, Jovanovic T, Norrholm SD (2015) Estrogen and extinction of fear memories: Implications for posttraumatic stress disorder treatment. Biol Psychiatry 78:178–185
Graham BM, Li SH, Black MJ, Öst LG (2018) The association between estradiol levels, hormonal contraceptive use, and responsiveness to one-session-treatment for spider phobia in women. Psychoneuroendocrinology 90:134–140
Graham BM, Milad MR (2013) Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry 73:371–378
Hagedorn B, Wolf OT, Merz CJ (2021) Stimulus-based extinction generalization: Neural correlates and modulation by cortisol. Int J Neuropsychopharmacol 4:354–365
Hamm A (2006) Spezifische Phobien. Fortschritte der Psychotherapie: Bd. 27. Hogrefe
Hautzinger M, Keller F, Kühner C (2006) Beck Depressions-Inventar (BDI-II). Revision. Pearson
Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B (2016) Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 6:603–621
Hofmann SG, Smits JAJ (2008) Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo controlled trials. J Clin Psychiatry 69:621–632
Hsu CM, Ney LJ, Honan C, Felmingham KL (2021) Gonadal steroid hormones and emotional memory consolidation: A systematic review and meta-analysis. Neurosci Biobehav Rev 130:529–542
Jentsch VL, Pötzl L, Wolf OT, Merz CJ (2022) Hormonal contraceptive usage influences stress hormone effects on cognition and emotion. Front Neuroendocrinol 67:101012
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602
Kirschbaum C, Hellhammer DH (1994) Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 19:313–333
Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ’Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
Kirschbaum C, Platte P, Pirke KM, Hellhemmer D (1996) Adrenocortical activation following stressful exercise: Further evidence for attenuated free cortisol responses in women using oral contraceptives. Stress Med 12:137–143
Kirschbaum C, Pirke KM, Hellhammer DH (1995) Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology 20:509–514
Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C (2004) HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology 29:83–98
Kudielka BM, Hellhammer DH, Wüst S (2009) Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34:2–18
Lass-Hennemann J, Michael T (2014) Endogenous cortisol levels influence exposure therapy in spider phobia. Behav Res Ther 60:39–45
Laux L (1981) Das State-Trait-Angstinventar (STAI): theoretische Grundlagen und Handanweisung. Beltz
Maeng LY, Milad MR (2015) Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav 76:106–117
Matchett G, Davey GC (1991) A test of a disease-avoidance model of animal phobias. Behav Res Ther 29:91–94
McLean CP, Asnaani A, Litz BT, Hofmann SG (2011) Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45:1027–1035
Meir Drexler S, Hamacher-Dang TC, Wolf OT (2017) Stress before extinction learning enhances and generalizes extinction memory in a predictive learning task. Neurobiol Learn Mem 141:143–149
Meir Drexler S, Merz CJ, Wolf OT (2018) Preextinction stress prevents context-related renewal of fear. Behav Ther 49:1008–1019
Meir Drexler S, Merz CJ, Jentsch VL, Wolf OT (2019) How stress and glucocorticoids timing-dependently affect extinction and relapse. Neurosci Biobehav Rev 98:145–153
Merz CJ, Kinner VL, Wolf OT (2018) Let's talk about sex … differences in human fear conditioning. Curr Opin Behav Sci 23: 7–12
Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT (2012) Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav 62:531–538
Merz CJ, Wolf OT (2017) Sex differences in stress effects on emotional learning. J Neurosci Res 95:93–105
Montero-López E, Santos-Ruiz A, García-Ríos MC, Rodríguez-Blázquez M, Rogers HL, Peralta-Ramírez MI (2018) The relationship between the menstrual cycle and cortisol secretion: Daily and stress-invoked cortisol patterns. Int J Psychophysiol 131:67–72
Mystkowski JL, Craske MG, Echiverri AM, Labus JS (2006) Mental reinstatement of context and return of fear in spider-fearful participants. Behav Ther 37:49–60
Pittig A, Treanor M, LeBeau RT, Craske MG (2018) The role of associative fear and avoidance learning in anxiety disorders: Gaps and directions for future research. Neurosci Biobehav Rev 88:117–140
Pletzer B, Poppelaars ES, Klackl J, Jonas E (2021) The gonadal response to social stress and its relationship to cortisol. Stress (amsterdam, Netherlands) 24:866–875
Pössel P, Hautzinger M (2003) Dysfunktionale Überzeugungen bei Spinnenangst. Z Klin Psychol Psychopathol Psychother 32:24–30
Preusser F, Margraf J, Zlomuzica A (2017) Generalization of extinguished fear to untreated fear stimuli after exposure. Neuropsychopharmacol 42:2545–2552
Raeder F, Heidemann F, Schedlowski M, Margraf J, Zlomuzica A (2019a) No pills, more skills: The adverse effect of hormonal contraceptive use on exposure therapy benefit. J Psychiatr Res 119:95–101
Raeder F, Merz CJ, Tegenthoff M, Wolf OT, Margraf J, Zlomuzica A (2019b) Post-exposure cortisol administration does not augment the success of exposure therapy: A randomized placebo-controlled study. Psychoneuroendocrinology 99:174–182
Raeder F, Woud ML, Schneider S, Totzeck C, Adolph D, Margraf J, Zlomuzica A (2019c) Reactivation and evaluation of mastery experiences promotes exposure benefit in height phobia. Cogn Ther Res 43(5):948–958
Raeder F, Merz CJ, Margraf J, Zlomuzica A (2020) The association between fear extinction, the ability to accomplish exposure and exposure therapy outcome in specific phobia. Sci Rep 10:4288
Regier DA, Narrow WE, Rae DS (1990) The epidemiology of anxiety disorders: The epidemiologic catchment area (ECA) experience. J Psychiatr Res 24:3–14
Rinck M, Bundschuh ST, Engler S, Müller A, Wissmann J, Ellwart T, Becker E (2002) Reliability and validity of German versions of three instruments measuring fear of spiders. Reliabilität und Validität dreier Instrumente zur Messung von Angst vor Spinnen [Reliability and validity of German versions of three instruments measuring fear of spiders.]. Diagnostica 48:141–149
Rowe M, Craske M (1998a) Effects of an expanding-spaced vs massed exposure schedule on fear reduction and return of fear. Behav Res Ther 36:701–717
Rowe MK, Craske MG (1998b) Effects of varied-stimulus exposure training on fear reduction and return of fear. Behav Res Ther 36:719–734
Scandola M, Bastianelli A, Spoto A, Vidotto G (2010) The Fear of Cockroaches Questionnaire (FCQ). Ann l Rev Psych 17:111–118
Schwabe L, Haddad L, Schachinger H (2008) Hpa axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 33:890–895
Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, De Quervain DJF (2006) Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci USA 103:5585–5590
Soravia LM, Heinrichs M, Winzeler L, Fisler M, Schmitt W, Horn H, Dierks T, Strik W, Hofmann SG, De Quervain DJF (2014) Glucocorticoids enhance in vivo exposure-based therapy of spider phobia. Depress Anxiety 31:429–435
Stinson FS, Dawson DA, Patricia Chou S, Smith S, Goldstein RB, June Ruan W, Grant BF (2007) The epidemiology of DSM-IV specific phobia in the USA: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med 37:1047–1059
Stockhorst U, Antov MI (2016) Modulation of Fear Extinction by Stress, Stress Hormones and Estradiol: A Review. Front Behav Neurosci 9:359
van Eck M, Berkhof H, Nicolson N, Sulon J (1996) The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med 58(5):447–458
Vervliet B, Baeyens F, Van den Bergh O, Hermans D (2013) Extinction, generalization, and return of fear: A critical review of renewal research in humans. Biol Psychol 92:51–58
Wardenaar KJ, Lim CCW, Al-Hamzawi AO, Alonso J, Andrade LH, Benjet C, Bunting B, De Girolamo G, Demyttenaere K, Florescu SE, Gureje O, Hisateru T, Hu C, Huang Y, Karam E, Kiejna A, Lepine JP, Navarro-Mateu F, Oakley Browne M, Piazza M, Posada-Villa J, Ten Have ML, Torres Y, Xavier M, Zarkov Z, Kessler RC, Scott KM, de Jonge P (2017) The cross-national epidemiology of specific phobia in the World Mental Health Surveys. Psychol Med 47:1744–1760
Waters AM, Ryan KM, Luck CC, Craske MG, Lipp OV (2021) The effects of presenting additional stimuli resembling the CS+ during extinction on extinction retention and generalisation to novel stimuli. Behav Res Ther 144:103921
White EC, Graham BM (2016) Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiol Learn Mem 134:339–348
Zlomuzica A, Preusser F, Schneider S, Margraf J (2015) Increased perceived self-efficacy facilitates the extinction of fear in healthy participants. Front Behav Neurosci 9:270
Zlomuzica A, Schneider S, Konrad C, Merz CJ, Wolf OT, Raeder F, Margraf J (2020) Clinical implications of fear extinction in anxiety disorders. Neuroforum 26:143–149
Acknowledgements
The study was supported by grants from the Deutsche Forschungsgemeinschaft (German Research Foundation; SFB 874, project A1 to M.T.; SFB 1280 Extinction Learning project SFB1280 – project number 316803389, Project A13 awarded to A.Z. and J.M., project A09 to C.J.M. and O.T.W.; Heisenberg Grant ZL 59 4–1 and ZL 59 5–1 awarded to A.Z.). The funders had no role in study design, data collection and analysis, preparation of the manuscript and decision to publish.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Friederike Raeder: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing—Original Draft. Armin Zlomuzica: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Review & Editing, Supervision. Ekrem Dere: Formal analysis, Writing—Review & Editing. Christian J. Merz: Conceptualization, Writing—Review & Editing. Martin Tegenthoff: Writing—Review & Editing, Oliver T. Wolf: Conceptualization, Writing—Review & Editing. Jürgen Margraf: Conceptualization, Writing—Review & Editing. Silvia Schneider: Conceptualization, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raeder, F., Merz, C.J., Tegenthoff, M. et al. Do oral contraceptives modulate the effects of stress induction on one-session exposure efficacy and generalization in women?. Psychopharmacology 240, 1075–1089 (2023). https://doi.org/10.1007/s00213-023-06345-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06345-3