Abstract
Rationale
Kratom (Mitragyna speciosa Korth), a native medicinal plant of Southeast Asia, is proposed to exhibit potential therapeutic value as an opioid substitute. However, studies of its negative emotional states resulting from withdrawal particularly of its main psychoactive compound, mitragynine (MG), are limited.
Objectives
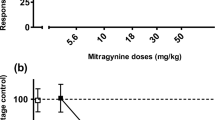
Using the pentylenetetrazol (PTZ) discrimination assay, this study aims to investigate the effects of MG in responding to the PTZ stimulus and to assess the generalisation effects of withdrawal from MG to the PTZ stimulus.
Methods
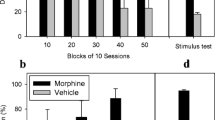
Rats (n = 20) were trained on a tandem (FR-10, VI-15) schedule of food reinforcement to press one lever after administration of the anxiogenic compound PTZ (16 mg/kg, i.p.) and an alternate lever after vehicle. Following acute tests, training was suspended, and rats were chronically treated with MG or morphine at 8-h intervals for 9 days and withdrawal was precipitated on the tenth day using naloxone (1 mg/kg, i.p.). The rats were tested for generalisation to PTZ at 2, 8 and 24 h after the last dose of MG or morphine administration.
Results
Unlike morphine that produced dose-related PTZ-like stimulus, MG at 3, 10, 30 and 45 mg/kg doses showed no substitution to the PTZ discriminative stimulus. In contrast to morphine which produced a time-dependent generalisation to the PTZ stimulus, naloxone did not precipitate withdrawal effects in MG-treated rats as they selected the vehicle lever at three withdrawal time points.
Conclusion
These results demonstrate that MG produces a very different response to morphine withdrawal that is not associated with anxiogenic-like subjective symptoms. These characteristics of MG may provide further support for use as a novel pharmacotherapeutic intervention for managing opioid use disorder.



Similar content being viewed by others
References
Adkins JE, Boyer EW, McCurdy CR (2011) Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr Top Med Chem 11:1165–1175
Ahmad K, Aziz Z (2012) Mitragyna speciosa use in the northern states of Malaysia: across-sectional study. J Ethnopharmacol 141:446–450
Ayhan IH, Randrup A (1973) Behavioural and pharmacological studies on morphine-induced excitation of rats Possible Relation to Brain Catecholamines. Psychopharmacologia 29(317):328
Becker GL, Gerak LR, Li J, Koek W, France CP (2010) Precipitated and conditioned withdrawal in morphine-treated rats. Psychopharmacology 209(1):85–94
Bronson ME (1993) Withdrawal from chronic haloperidol substitutes for the pentylenetetrazol discriminative stimulus. Life Sci 52:129–133
Bronson ME, Roberts J (1992) Withdrawal from chronic phencyclidine produces a pentylenetetrazol-like discriminative stimulus. Life Sci 50:499–504
Coe MA, Pillitteri JL, Sembower MA, Gerlach KK, Henningfield JE (2019) Kratom as a substitute for opioids: results from an online survey. Drug Alcohol Depend 202:24–32
Cools AR, Janssen HJ, Broekkamp CLE (1974) The differential role of the caudate nucleus and the linear raphe in the initiation and the maintenance of morphine-induced behaviour in cats. Arch Int Pharmacodyn 210:163–174
Eastlack SC, Cornett EM, Kaye AD (2020) Kratom - pharmacology, clinical implications and outlook: a comprehensive review. Pain Ther 9:55–69
El-Kadi AOS, Sharif SI (1994) The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. Gen Pharmacol 25:1505–1510
Emmett-Oglesby MW, Harris CM, Lane JD, Lal H (1984) Withdrawal from morphine generalizes to a pentylenetetrazol stimulus. Neuropeptides 5:37–40
Emmett-Oglesby MW, Herz A (1987) Opioid modulation of the discriminative stimulus produced by pentylenetetrazol. Psychopharmacology 92:313–319
Emmett-Oglesby MW, Mathis DA, Lal H (1987) Diazepam tolerance and withdrawal assessed in an animal model of subjective drug effects. Drug Dev Res 11:145–156
Emmett-Oglesby MW, Rowan GA (1991) Drug discrimination used to study drug withdrawal. NIDA Res Monogr 116:337–357
Farah Idayu N, Taufik Hidayat M, Moklas MAM, Sharida F, Nurul Raudzah AR, Shamima AR, Apryani E (2011) Anti-depressant like effect of mitragynine isolated from Mitragyna speciosa korth in mice model of depression. Phytomedicine 18:402–407
Garcia-Romeu A, Cox DJ, Smith KE, Dunn KE, Griffiths RR (2020) Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend 208:107849
Gauvin DV, Harland RD, Holloway FA (1989) Drug discrimination procedures: a method to analyse adaptation level of affective states. Drug Dev Res 16:183–194
Gatch MB, Wallis CJ, Lal H (2000) Effects of ritanserin on ethanol withdrawal-induced anxiety in rats. Alcohol 21:11–17
Grundmann O (2017) Patterns of kratom use and health impact in the US - results from an online survey. Drug Alcohol Depend 176:63–70
Harris CM, Emmett-Oglesby MW, Robinson NG, Lal H (1986) Withdrawal from chronic nicotine substitutes partially for the interoceptive stimulus produced by pentylenetetrazol (PTZ). Psychopharmacology 90:85–89
Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M (2015) Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology 232(13):2227–2238
Harun N, Johari IS, Mansor SM, Shoaib M (2020) Assessing physiological dependence and withdrawal potential of mitragynine using schedule-controlled behaviour in rats. Psychopharmacology 237:855–867
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NHM, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NIW, Jayabalan N, Hazim AI, Mansor SM, Müller CP (2013) From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 37:138–151
Hassan R, Pike See C, Sreenivasan S, Mansor SM, Müller CP, Hassan Z (2020) Mitragynine attenuates morphine withdrawal effects in rats-a comparison with methadone and buprenorphine. Front Psychiatry 7(11):411
Hazim AI, Ramanathan S, Parthasarathy S, Muzaimi M, Mansor SM (2014) Anxiolytic-like effects of mitragynine in the open-field and elevated plus-maze tests in rats. J Physiol Sci 64:161–169
Hemby SE, McIntosh S, Leon F, Cutler SJ, McCurdy CR (2019) Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict Biol 24(5):874–885
Henningfield JE, Fant RV, Wang DW (2018) The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology 235(2):573–589
Johnson LE, Balyan L, Magdalany A, Saeed F, Salinas R, Wallace S, Veltri CA, Swogger MT, Walsh Z, Grundmann O (2020) The potential for kratom as an antidepressant and antipsychotic. Yale J Biol Med 93(2020):283–289
Jones CM, Einstein EB, Compton WM (2018) Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. JAMA 319(17):1819–1821
Jung ME, Wallis CJ, Gatch MB, Lal H (1999) Sex differences in the pentylenetetrazol-like stimulus induced by ethanol withdrawal. J Pharmacol Exp Ther 292:576–582
Jung ME, Lal H, Gatch MB (2002) The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments. Neurosci Biobehav Rev 26:429–439
Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS (2002) Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience 115(2):463–469
Kim S (2019) The unsuspected threat of three opioid-like substitutes. Arch Psychiat Nurs 33:325–328
Koob G, Le Moal M (2005a) Neurobiology of addiction. Academic press, San Diego
Koob G, Le Moal M (2005b) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8:1442–1444
Kruegel AC, Grundmann O (2018) The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 134:108–120
Lal H, Emmett-Oglesby MW (1983) Behavioural analogues of anxiety. Anim Models Neuropharmacol 22:1423–1441
Leong Bin Abdullah MFI, Singh D, Swogger MT, Rahim AA, Vicknasingam B (2019) The prevalence of psychotic symptoms in kratom (Mitragyna speciosa korth.) users in Malaysia. Asian J Psychiatr 43:197–201
Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, Watanabe H (1996) Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci 59:1149–1155
Meepong R, Sooksawate T (2019) Mitragynine reduced morphine-induced conditioned place preference and withdrawal in rodents. Thai J Pharm Sci 43(1):21–29
O’Brien CP (1996) Drug addiction and drug abuse. In: Hardman JG, Limbird LE (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw-Hill, New York, pp 557–577
Petrosky E, Harpaz R, Fowler KA, Bohm MK, Helmick CG, Yuan K, Betz CJ (2018) Chronic pain among suicide decedents, 2003 to 2014: findings from the National Violent Death Reporting System. Ann Intern Med 169(7):448–455
Prather PL, Lal H (1992) Protracted withdrawal: sensitization of the anxiogenic response to cocaine in rats concurrently treated with ethanol. Neuropsychopharmacology 6:23–29
Prozialeck WC, Jivan JK, Andurkar SV (2012) Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc 112(12):792–799
Prozialeck WC, Avery BA, Boyer EW, Grundmann O, Henningfield JE, Kruegel AC, McMahon LR, McCurdy CR, Swogger MC, Veltri CA, Singh D (2019) Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy 70:70–77
Saingam SD, Assanangkornchai S, Geater AF, Balthip Q (2013) Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Pol 24:351–358
Sasaki K, Fan LW, Tien LT, Ma T, Loh HH, Ho IK (2002) The interaction of morphine and gamma-aminobutyric acid (GABA)ergic systems in anxiolytic behavior: using mu-opioid receptor knockout mice. Brain Res Bull 57:689–694
Self DW, Nestler EJ (1998) Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend 51(1–2):49–60
Seth P, Scholl L, Rudd RA, Bacon S (2018) Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. Am J Transplant 18(6):1556–1568
Singh D, Müller CP, Vicknasingam BK (2014) Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend 139:132–137
Singh D, Müller CP, Vicknasingam BK, Mansor SM (2015) Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psycho Drugs 47(2):125–131
Singh D, Narayanan S, Vicknasingam B, Corazza C, Santacroce R, Roman-Urrestarazu A (2017) Changing trends in the use of kratom (Mitragyna speciosa) in Southeast Asia. Hum Psychopharmacol Clin Exp 32:e2582
Singh D, Narayanan S, Müller CP, Swogger MT, Rahim AA, Leong Bin Abdullah MFI, Vicknasingam BK (2018) Severity of kratom (Mitragyna speciosa Korth.) psychological withdrawal symptoms. J Psycho Drugs 50(5):445–450
Swogger MT, Hart E, Erowid F, Trabold N, Yee K, Parkhurst KA, Priddy BM, Walsh Z (2015) Experiences of kratom users: a qualitative analysis. J Psycho Drugs 47(5):360–367
Swogger MT, Walsh Z (2018) Kratom use and mental health: a systematic review. Drug Alcohol Depend 183:134–140
Tsuda M, Suzuki T, Misawa M, Nagase H (1996) Involvement of the opioid system in the anxiolytic effect of diazepam in mice. Eur J Pharmacol 307:7–14
Utar Z, Majid MI, Adenan MI, Jamil MF, Lan TM (2011) Mitragynine inhibits the COX-2 mRNA expression and prostaglandin E (2) production induced by lipopolysaccharide in RAW264.7 macrophage cells. J Ethnopharmacol 136:75–82
Uzbay IT, Lal H (2002) Effects of Ng-nitro-L-arginine methyl ester, 7-nitro indazole, and agmatine on pentylenetetrazol-induced discriminative stimulus in Long-Evans rats. Prog Neuropsychopharmacol Biol Psychiatr 26:567–573
Vijeepallam K, Pandy V, Kunasegaran T, Murugan DD, Naidu M (2016) Mitragyna speciosa leaf extract exhibits antipsychotic-like effect with the potential to alleviate positive and negative symptoms of psychosis in mice. Front Pharmacol 7:464
Ward J, Rosenbaum C, Hernon C, McCurdy CR, Boyer EW (2011) Herbal medicines for the management of opioid addiction: safe and effective alternatives to conventional pharmacotherapy. CNS Drugs 25:999–1007
Yusoff NH, Suhaimi FW, Vadivelu RK, Hassan Z, Rümler A, Rotter A, Amato D, Dringenberg HC, Mansor SM, Navaratnam V, Müller CP (2016) Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol 21(1):98–110
Funding
This research was financially supported by USM Short Term Research Grant (304/CDADAH/6315105), Higher Education Centre of Excellence (HiCoE) special funding (311/CDADAH/4401009) and Yang di-Pertuan Agong (BYDPA) scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Johari, I.S., Harun, N., Sofian, Z.M. et al. Pentylenetetrazol-like stimulus is not produced following naloxone-precipitated mitragynine withdrawal in rats. Psychopharmacology 238, 3183–3191 (2021). https://doi.org/10.1007/s00213-021-05934-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05934-4