Abstract
Rationale
Kratom is proposed to exhibit therapeutic potential as an opium substitute, but little is known about its dependence-producing profile, particularly of its main psychoactive compound, mitragynine (MG).
Objectives
This study examined the dependence-producing effects of MG using operant-scheduled behaviour in rats and investigated the potential therapeutic effect of MG by comparing effects to buprenorphine in morphine-dependent rats using the same schedule-controlled behavioural task.
Methods
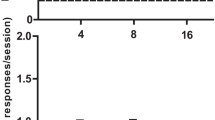
The effects of acutely administered MG and morphine were determined in rats trained to respond under fixed-ratio (FR) 10 schedule of food reinforcement. Next, the rats were administered MG and morphine twice daily for 14 consecutive days to determine if physiological dependence would develop by examining cessation of drug treatment and following antagonist-precipitated withdrawal. The study then examined the effects of MG substitution to suppress naloxone-precipitated morphine withdrawal effects on scheduled responding.
Results
Acute doses of MG did not produce dose-related decreases on FR schedules of responding compared to morphine. Unlike morphine, MG-treated rats showed no suppression of response rates following cessation of MG treatment. However, withdrawal effects were evident for MG after precipitation by either naloxone or SR141716A (rimonabant), similar to morphine-treated rats. MG in higher doses (10 and 30 mg/kg) attenuated the naloxone-precipitated morphine withdrawal effects while smaller doses of buprenorphine (0.3 and 1.0 mg/kg) were necessary to alleviate these effects.
Conclusion
The findings suggest that MG does not induce physiological dependence but can alleviate the physical symptoms associated with morphine withdrawal which represent the desired characteristics of novel pharmacotherapeutic interventions for managing opioid use disorder (OUD).





Similar content being viewed by others
References
Adams JU, Holtzman SG (1990) Tolerance and dependence after continuous morphine infusion from osmotic pumps measured by operant responding in rats. Psychopharmacology 100(4):451–458
Aceto MD (1990) Assessment of physical dependence techniques for the evaluation of abused drugs. In: Adler MW, Cowan A (eds) Testing and evaluation of drugs of abuse. Wiley-Liss, New York, pp 67–79
Ahmad K, Aziz Z (2012) Mitragyna speciosa use in the northern states of Malaysia: a cross-sectional study. J Ethnopharmacol 141:446–450
An XF, Zhang Y, Winter JC, Li JX (2012) Effects of imidazoline I2 receptor agonists and morphine on schedule-controlled responding in rats. Pharmacol Biochem Behav 101(3):354–359
Anraku T, Ikegaya Y, Matsuki N, Nishiyama N (2001) Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology 157:217–220
Assanangkornchai S, Muekthong A, Sam-Angsri N, Pattanasattayawong U, Takayama H (2007) The use of Mitragyna speciosa (“Krathom”), an addictive plant, in Thailand. Subst Use Misuse 42:2145–2157
Balster RL (1985) Behavioural studies of tolerance and dependence. In: Seidin LS, Balster RL (eds) Behavioural pharmacology; the current status. Neurology and neurobiology, vol 13. Liss, New York, pp 403–418
Beardsley PM, Martin BR (2000) Effects of the cannabinoid CB1 receptor antagonist, SR141716A, after Δ9-tetrahydrocannabinol withdrawal. Eur J Pharmacol 387(1):47–53
Becker GL, Gerak LR, Li J, Koek W, France CP (2010) Precipitated and conditioned withdrawal in morphine-treated rats. Psychopharmacology 209(1):85–94
Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH (2008) Self-treatment of opioid withdrawal using kratom (Mitragyna speciosa korth). Addiction 103(6):1048–1050
Cheaha D, Kewapradub N, Sawangjaroen K, Phukpattaranont P, Kumarnist E (2017) Effects of an alkaloid-rich extract from Mitragyna speciosa leaves and fluoxetine on sleep profiles, EEG spectral frequency and ethanol withdrawal symptoms in rats. Phytomed 22:1000–1008
Cooper ZD, Troung YN, Shi YG, Woods JH (2008) Morphine deprivation increases self-administration of the fast- and short- acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther 326:920–929
Cooper ZD, Shi YG, Woods JH (2010) Reinforcer-dependent enhancement of operant responding in opioid withdrawn rats. Psychopharmacology 212:369–378
Compton WM, Volkow ND (2006) Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81(2):103–107
Fride E (2002) Endocannabinoids in the central nervous system- an overview. Prostaglandins Leukot Essent Fatty Acids 66(2–3):221–233
Ford RD, Balster RL (1976) Schedule-controlled behaviour in the morphine-dependent rat. Pharmacol Biochem Behav 4:569–573
Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS (1989) Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology 97:295–302
Gonzalez G, Oliveto A, Kosten TR (2004) Combating opiate dependence: a comparison among the available pharmacological options. Expert Opin Pharmacother 5:4,713–4,725
Grundmann O (2017) Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend 176:63–70
Gunter BW, Platt DM, Rowlett JK (2015) Differential interactions engendered by benzodiazepine and neuroactive steroid combinations on schedule-controlled responding in rats. Pharm Biochem and Behav 137:53–59
Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M (2015) Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology 232(13):2227–2238
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NI, Jayabalan N, Hazim AI, Mansor SM, Müller CP (2013) From kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse and addiction. Neurosci Biobehav Rev 37(2):138–151
Hemby SE, McIntosh S, Leon F, Cutler SJ, McCurdy CR (2018) Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict Biol. https://doi.org/10.1111/adb.12639
Hiranita T, Leon F, Felix JS, Rastrepo LF, Reeves ME, Pennington AE, Obeng S, Avery BA, McCurdy CR, McMahon LR, Wilkerson JL (2019) The effects of mitragynine and morphine on scheduled-controlled responding and antinociception in rats. Psychopharmacology 236:2725–2734. https://doi.org/10.1007/s00213-019-05247-7
Ismail NIW, Jayabalan N, Mansor SM, Muller CP, Muzaimi M (2017) Chronic mitragynine (kratom) enhances punishment resistance in natural reward seeking and impairs place learning in mice. Addict Biol 22(4):967–976
Jones JD, Manubay JM, Mogali S, Metz VE, Madera G, MartinezS MM, Comer SD (2017) Abuse liability if intravenous buprenorphine vs. buprenorphine/naloxone: importance of absolute naloxone amount. Drug Alcohol Depend 179:362–369
Khor B, Jamil S, Adenan MFA, Adenan MI, Shu-Chien AC (2011) Mitragynine attenuates withdrawal syndrome in morphine-withdrawn zebrafish. PLoS One 6(12):e28340
Kruegel AC, Gassaway MM, Kapoor A, Varadi A, Majumdar S, Filizola M (2016) Synthetic and receptor signaling explorations of the Mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J American Chem Soc 138:6754–6764
Li X, Li JX, France CP (2010) Interactions between morphine, scopolamine and nicotine: schedule-controlled responding in rats. Pharmacol Biochem Behav 96(1):91–95
Lichtman AH, Sheikh SM, Loh HH, Martin BR (2001) Opioid and cannabinoid modulation of precipitated withdrawal in Δ9-Tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther 298:1007–1041
Macko E, Weisbach JA, Douglas B (1972) Some observations on the pharmacology of mitragynine. Arch Int de Pharmacodynamieet de Therapie 198:145–161
Maldonado R (2002) Study of cannabinoid dependence in animals. Pharmacol Ther 95:153–164
Maldonado R, Valverde O, Berrendero F (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29(4):225–232
Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, Watanabe H (1996) Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci 59:1149–1155
Nanthini J, Ismail NIW, Mansor SM, Muller CP, Muzaimi M (2015) Cerebellum and endocannabinoid receptors: a new possible neurobiological link for mitragynine (Mitragyna speciosa Korth) abuse liability. J Addict Depend 1(1):1–7
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharma 7:27–31
Navarro M, Chowen J, Carrera MRA, del Arco I, Villanua MA, Martin Y, Robert AJ, Koob GF, de Fonseca FR (1998) CB1 cannabinoid receptor antagonist induced opiate withdrawal in morphine-dependent rats. Neuroreport 9:3397–3402
Palma J, Abood ME, Benamar K (2015) HIV-gp120 and physical dependence to buprenorphine. Drug Alcohol Depend 150:175–178
Prozialeck WC, Jivan JK, Andurkar SV (2012) Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc 112(12):792–799
Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, Aimi N, Sakai S (1994) A new indole alkaloid, 7alpha-hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand. Planta Med 60:580–581
Sabetghadam A, Navaratnam V, Mansor SM (2013) Dose-response relationships, acute toxicity, and therapeutic index between the alkaloid extract of Mitragyna speciosa and its main active compound mitragynine in mice. Drug Dev Res 74:23–30
Saingam D, Assanangkornchai S, Geater AF, Balthip Q (2013) Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy 24(4):351–358
Salmanzadeh H, Azizi H, Semnanian S (2017) Adolescent chronic escalating morphine induce long lasting changes in tolerance and dependence to morphine in rats. Physio Behav 174:191–196
Schulties G, Morse AC, Liu J (2003) Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav 76:493–503
Shamima AR, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MA, Arulsevan P (2012) Antinociceptive action of isolated mitragynine from Mitragyna speciosa through activation of opioid receptor system. Int J Mol Sci 13:11427–11442
Singh D, Müller CP, Vicknasingam BK (2014) Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend 139:132–137
Singh D, Narayanan S, Vicknasingam B (2016) Traditional and non-traditional uses of mitragynine (Kratom): a survey of the literature. Brain Res Bull 126:41–46
Singh D, Narayanan S, Vicknasingam B, Corazza C, Santacroce R, Roman-Urrestarazu A (2017) Changing trends in the use of kratom (Mitragyna speciosa) in Southeast Asia. Hum Psychopharmacol Clin Exp:e2582
Shellard EJ (1974) The alkaloids of Mitragyna with special reference to those of Mitragyna speciosa Korth. Bull Narc 26(2):41–55
Stoller DC, Smith FL (2004) Buprenorphine blocks withdrawal in morphine-dependent rat pups. Pediatr Anesth 14:642–649
Suwanlert S (1975) Study of kratom eaters in Thailand. Bull Narc 27(3):21–27
Swogger MT, Hart E, Erowid F, Trabold N, Yee K, Parkhurst KA, Priddy BM, Walsh Z (2015) Experiences of kratom users: a qualitative analysis. J Psycho Drugs 47(5):360–367
Swogger MT, Walsh Z (2018) Kratom use and mental health: a systematic review. Drug Alcohol Depend 183:134–140
Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S (2002) Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands. J Med Chem 45:1949–1956
Thongpradichote S, Matsumoto K, Tohda M, Takayama H, Aimi N, Sakai S, Watanabe H (1998) Identification of opioid receptor subtypes in antinociceptive actions of supraspinally-administered mitragynine in mice. Life Sci 62:1371–1378
Thorn DA, Zhang Y, Li J (2016) Effects of the imidazoline I2 receptor agonist 2-BFI on the development of tolerance to and behavioural/physical dependence on morphine in rats. Br J Pharmacol 173:1363–1372
Tsou K, Patrick SL, Walker JM (1995) Physical withdrawal in rats tolerant to Δ9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol 280(3):R13–R15
Utar Z, Majid MI, Adenan MI, Jamil MF, Lan TM (2011) Mitragynine inhibits the COX-2 mRNA expression and prostaglandin E (2) production induced by lipopolysaccharide in RAW264.7 macrophage cells. J Ethnopharmacol 136:75–82
Varvel SA, Vann RE, Wise LE, Philibin SD, Porter JH (2002) Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology 160:182–191
Vicknasingam B, Narayanan S, Beng GT, Mansor SM (2010) The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy 21:283–288
Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic advances from brain disease model of addiction. N Engl J Med 374(4):363–371
Watanabe K, Yano S, Horie S, Yamamoto LT, Sakai S, Takayama H, Ponglux D, Wongseripipatan S (1992) Pharmacological profiles of ‘kratom’ (Mitragyna speciosa), a Thai medical plant with special reference to its analgesic activity. In: Tongroach P, Watanabe H, Ponglux D, Suvanakoot U, Ruangrungsi N (Eds.). Adv Res Pharmacol Act Subst Nat Prod 125-132
Watanabe K, Yano S, Horie S, Yamamoto LT, Takayama H, Aimi H, Sakai S, Ponglux D, Tongroacj P, Shan J, Pang PKT (1999) Pharmacological properties of some structurally related indole alkaloids contained in the Asian herbal medicines, hirsutine and mitragynine, with special reference to their Ca2+ antagonistic and opioid-like effects. In: Watanabe, H., Shibuya, N.R., Farnsworth, N.R. (Eds.). Pharmacol Res Tradit Herb Med 11:163–177
Yue K, Kopajtic TA, Katz JL (2018) Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology 235(10):2823–2829
Yusoff NH, Suhaimi FW, Vadivelu RK, Hassan Z, Rümler A, Rotter A, Amato D, Dringenberg HC, Mansor SM, Navaratnam V, Müller CP (2016) Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol 21(1):98–110
Yusoff NH, Mansor SM, Müller CP, Hassan Z (2017) Opioid receptors mediate the acquisition, but not the expression of mitragynine-induced conditioned place preference in rats. Behav Brain Res 332:1–6
Funding
This research received financial support from Higher Education Centre of Excellence (HICoE) special funding (311/CDADAH/4401009) and USM Short Term Research Grant (304/CDADAH/6315105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were conducted within the bounds of local ethical regulations and were carried out in accordance with the guidelines for the use of experimental animals and approved by the Animal Ethics Committee, Universiti Sains Malaysia (AECUSM).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Harun, N., Johari, I.S., Mansor, S.M. et al. Assessing physiological dependence and withdrawal potential of mitragynine using schedule-controlled behaviour in rats. Psychopharmacology 237, 855–867 (2020). https://doi.org/10.1007/s00213-019-05418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05418-6