Abstract
Rationale
Management of anxiety, delirium, and agitation cannot be neglected in coronavirus disease (COVID-19). Antipsychotics are usually used for the pharmacological management of delirium, and confusion and behavioral disturbances. The concurrent use of treatments for COVID-19 and antipsychotics should consider eventual drug-drug interactions
Objective
To systematically review evidence-based available on drug-drug interactions between COVID-19 treatments and antipsychotics.
Evidence review
Three databases were consulted: Lexicomp® Drug Interactions, Micromedex® Solutions Drugs Interactions, and Liverpool© Drug Interaction Group for COVID-19 therapies. To acquire more information on QT prolongation and Torsade de Pointes (TdP), the CredibleMeds® QTDrugs List was searched. The authors made a recommendation agreed to by consensus. Additionally, a systematic review of drug-drug interactions between antipsychotics and COVID-19 treatment was conducted.
Results
The main interactions between COVID-19 drugs and antipsychotics are the risk of QT-prolongation and TdP, and cytochromes P450 interactions. Remdesivir, baricinitib, and anakinra can be used concomitantly with antipsychotics without risk of drug-drug interaction (except for hematological risk with clozapine and baricinitib). Favipiravir only needs caution with chlorpromazine and quetiapine. Tocilizumab is rather safe to use in combination with antipsychotics. The most demanding COVID-19 treatments for coadministration with antipsychotics are chloroquine, hydroxychloroquine, azithromycin, and lopinavir/ritonavir because of the risk of QT prolongation and TdP and cytochromes interactions. The systematic review provides highly probable drug interaction between lopinavir/ritonavir plus quetiapine and ritonavir/indinavir plus risperidone.
Conclusions
Clinicians prescribing antipsychotics should be aware of the likely risk of drug-drug interaction with COVID-19 medication and may benefit from taking into account present recommendations of use to preserve patient safety
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of people affected by coronavirus disease 2019 (COVID-19) worldwide is over 32 M and rapidly increasing, and the importance of knowledge of symptom management and treatment for general practitioners and other clinicians must be stressed. Although the key symptoms are cough, fever, and breathlessness, anxiety, delirium, and agitation are also present in a large number of patients (National Institute for Health and Care Excellence (NICE) in collaboration with NHS England and NHS Improvement 2020). Up to 20–30% of COVID-19 patients will have with or develop delirium or changes in the mental health during their hospitalizations, and these numbers are as high as 60–70% in severely ill patients (Helms et al. 2020; Mao et al. 2020). The duration of delirium has consistently proven to be an independent predictor of longer hospitalization, higher mortality, and higher cost (E. Ely et al. 2001; E. W. Ely et al. 2004; Vasilevskis et al. 2018), and delirium is often inadequately managed (O’Hanlon and Inouye 2020). Antipsychotics drugs (APs) are usually selected for the pharmacological management of delirium (Kotfis et al. 2018), and confusion and behavioral disturbances associated with hospitalization of elderly patients (Jin and Liu 2019; van der Linde et al. 2014).
COVID-19 patients are being treated with chloroquine, hydroxychloroquine (Colson et al. 2020; Shukla et al. 2020), azithromycin (Gautret et al. 2020), and lopinavir/ritonavir (Cao et al. 2020), with a possible increased of QT prolongation and TdP, and other drug-drug interactions (DDIs) in coadministration with antipsychotics. The severely ill patients being treated are the most likely to suffer from QT prolongation, because of the clinical condition known to prolong QT, and the concomitant drugs, and clinicians need to mitigate that risk (Giudicessi et al. 2020). This use is complicated by age-related decline in drug metabolism (Zanger and Schwab 2013), high incidence of concomitant physical illnesses, drug-drug interactions, and heightened sensitivity to APs (Gareri et al. 2014; Maher et al. 2011; Rivière et al. 2019). In addition, COVID-19 also affects the cardiovascular system. Arrhythmia (atrial fibrillation, ventricular fibrillation, and ventricular tachyarrhythmia), cardiac injury, fulminant myocarditis, heart failure, pulmonary embolism, and disseminated intravascular coagulation are the most common cardiovascular complications in COVID-19 patients (Guzik et al. 2020); some of which are clinical factor associated with prolonged QTc and/or TdP (Woosley et al. n.d.-a).
APs are associated with a proarrhythmic state and an increased risk of sudden cardiac death (SCD), without substantial differences between first and second generation antipsychotics, and a dose-response effect (Ray et al. 2009; Salvo et al. 2016). Antipsychotics (exception aripiprazole and lurasidone) seem to be associated with a prolonged QT interval and an increased risk of SCD (Acciavatti et al. 2017). There are differences among APs in the degree of cardiotoxic effects (Leucht et al. 2013). SCD describes the unexpected natural death, from a cardiac cause within a short time, of a person who often has no prior cardiological condition that would appear fatal (Wellens et al. 2014). Many antipsychotics show a certain degree of blockade of potassium channels coded by the human ether-à-go-go-related gene (hERG), thus inducing a QT interval prolongation and increasing the risk of polymorphic ventricular tachycardia/torsade de Pointes (TdP) (Roden 2004; Testai et al. 2004). The most common acquired cause of long QT syndrome and TdP is drug-induced QT interval prolongation. Intensive care unit patients are particularly prone to experience a QTc interval prolongation mainly due to certain drugs that can prolong the repolarization phase, either by their action mechanism or through the interaction with other drugs (Etchegoyen et al. 2017) (a complete list of clinical factors associated with prolonged QTc and/or TdP can be consulted in www.QTFactors.org). Elderly patients are in particularly prone to proarrhythmic risk with antipsychotics (Vieweg et al. 2009). Also, the potential for pharmacokinetic DDIs of APs are mostly mediated by cytochrome P450 (CYP) enzymes metabolism; therefore, physicians should be aware of co-administered drugs that may inhibit or induce these CYP enzymes (Conley and Kelly 2007; Kennedy et al. 2013; King et al. 2004).
The aim of our review is to analyze the risks of DDIs and cardiovascular adverse events of antipsychotics with the main treatments currently used for COVID-19. More so, SARS-CoV-2 primarily affects the elderly with other associated cardiovascular risk factors, which often require hospitalization, and treatment of which is essential to knowing the risk and safety of antipsychotics associated with COVID-19 treatments.
Methods
Drugs currently used in the treatment of SARS-CoV-2 (Sanders et al. 2020) such as chloroquine, hydroxychloroquine, lopinavir/ritonavir, remdesivir, and tocilizumab have been reviewed and also azithromycin with promising results (Gautret et al. 2020), and potential therapeutic agents such as favipiravir (Jean et al. 2020), baricitinib (Cantini et al. 2020), and anakinra (Monteagudo et al. 2020).
Drug-drug interactions: antipsychotics and COVID19 drugs
Three databases were searched: (a) Lexicomp® Drug Interactions (Wolters Kluwer 2020), (b) Micromedex® Solutions Drugs Interactions (Truven Health Analytics. Micromedex® Solutions Drugs Interaction [Electronic Version], 2020), and (c) Liverpool© Drug Interaction Group for COVID-19 therapies (University of Liverpool. Liverpool COVID-19 Interactions, 2020). After an exhaustive review of various drugs interaction databases, the first two met the minimum quality criteria (Rodríguez-Terol et al. 2009), with good scores for scope, completeness, and ease of use (Patel and Beckett 2016). The following information was extracted (when available): type of interaction, risk rating, and severity (both, according to the stratification in each database) and patient management recommendations.
QT prolongation and TdP risk
To find out more about QT prolongation and TdP, the CredibleMeds® QTDrugs List (Woosley et al. 2020) was searched. CredibleMeds® classifies drugs based on their risk of QT prolongation or TdP—risk category: (a) Known Risk of TdP, (b) Possible Risk of TdP, (c) Conditional Risk of TdP.
Consensus recommendation
After the above information had been collected, the authors made consensus recommendations based on the following:
-
1.
Not recommended (red zone), if (1) coadministration contraindicated in one or more databases, or (2) both drugs classified as “Known Risk of TdP” (risk of QT prolongation or TdP).
-
2.
Recommended with caution. Two categories:
-
a)
Potential interaction, which may require a dose adjustment, close monitoring, or choosing alternative agents, in two or more databases (orange zone).
-
b)
Potential interaction intensity likely to be weak. Additional action/monitoring or dose adjustment unlikely to be required, in two or more databases (yellow zone).
-
3.
Recommended (green zone), if little or no evidence of clinically significant interaction is expected in two or more databases.
When there were differences between databases, and it was not possible to reach an agreement by consensus, our recommendations reflect the most conservative approach.
When QT-prolongation is the main risk, we present the APs in two groups according to CredibleMeds® (“Possible” or “Conditional” risk) to increase the clinical information.
Systematic review
Additionally, a systematic review was conducted according to PRISMA guidelines (Moher et al. 2009) (registration number in PROSPERO CRD42020183202) to find clinical outcomes of drug-drug interactions between COVID-19 treatment and antipsychotics.
We researched the following databases: MEDLINE, EMBASE, and Web of Science. The search was restricted to the English language using database filters. No date restriction was imposed for the updated search. The search strategy will follow syntax: antipsychotic* AND (chloroquine OR hydroxychloroquine OR lopinavir OR ritonavir OR remdesivir OR tocilizumab OR azithromycin OR favipiravir OR baricitinib OR anakinra). The reference list of all identified articles was examined for any additional study not found in the first search.
Inclusion and exclusion criteria
Inclusion criteria which refer to the characteristics for a given study to be eligible are as follows: (1) publication reporting clinically significant drug-drug interactions (between antipsychotics and COVID-19 treatment in adult patients (whatever the disease prescribed for) and (2) types of publication: randomized controlled trial, other controlled study, observational study, case report, and case series. Exclusion criteria are as follows: (1) only pharmacokinetic studies (no clinical outcome), (2) unpublished studies or gray literature and conference articles.
Three reviewers (G.R., A.R., M.R.) independently screened titles for the research question and criteria. When articles were deemed to meet the inclusion criteria by either reviewer, the abstract was analyzed. Full texts were retrieved when the reviewers were in agreement that the article met the inclusion criteria. Disagreements were solved by a fourth independent reviewer (B.P.). Rayyan QCR was used for duplicated data and screening (Ouzzani et al. 2016).
Data extraction
Two authors reached an agreement regarding the type of data to be collected, and relevant information such as the study design, context of drug interactions, clinical outcome, other comedication data, and the numbers and types of patients was recorded.
Risk of bias and generalizability concerns in comparative studies were evaluated with an adaptation of the Newcastle-Ottawa quality assessment scale for case control and cohort studies (Stang 2010), an eight-item tool designed to rate methodological aspects of case-control and cohort studies, which include three domains: study selection, comparability of cohorts on the basis of the design or analysis, and exposure. The overall score ranges from 0 to 9. Risk of bias and generalizability concerns in case reports and case series were evaluated with the Drug Interaction Probability Scale (DIPS) tool to assess the quality of papers retained (Horn et al. 2007). This 10-question tool evaluates drug interaction causation. Scores < 2 indicate doubtful drug interaction, scores 2–4 possible, 5–8 probable, and scores ≥ 9 indicate highly probable drug interaction.
Results
The authors’ consensus recommendations are summarized in Table 1.
Drug-drug interaction between APs and Chloroquine/Hydroxychloroquine/Azithromycin
The primary interaction is the risk of QT prolongation and TdP because the three drugs for COVID-19 have “Known Risk of TdP” according to CredibleMeds®.
Recommended use of APs with these treatments is summarized in Table 2.
The summary information about the risk rating, severity, and patient management for each database is available in Supplement Tables 1, 2, and 3.
The recommended concomitant APs for use with these 3 COVID-19 drugs are asenapine, cariprazine, brexpiprazole, and lurasidone. Olanzapine may be used in combination with hydroxychloroquine and azithromycin, and risperidone with azithromycin. Olanzapine with chloroquine has different risk rating: green in Liverpool© Drug Interaction Group, yellow in Lexicomp® Drug Interactions, and orange in Micromedex® Solutions Drugs Interactions database; for that reason, it is not possible to reach an agreement according to the author’s consensus and the recommendations reflect the most conservative approach, and so it’s necessary to take it with caution.
Drug-drug interaction between APs and lopinavir/ritonavir
This coadministration involves CYP interactions and the risk of QT prolongation and TdP. Lopinavir and ritonavir have “Possible Risk of TdP” according to CredibleMeds®. Lopinavir is metabolized primarily by hepatic CYP3A4 isoenzymes. Ritonavir inhibit CYP2D6 in vitro but to a lesser extent than CYP3A4, and numerous pharmacokinetics studies suggested that ritonavir is a CYP 3A, 1A2, 2B6, 2C9, 2C19, and glucuronidation inducer. And also, ritonavir is an inducer and inhibitor of p-glycoprotein. The coadministration of lopinavir/ritonavir helps to stave off lopinavir’s biotransformation and increased plasma levels of active antiviral drug (thanks to minimize the first pass metabolism). Due to this co-treatment between lopinavir and ritonavir, concomitant therapy with other medications which are CYP3A4 substrate can lead to increased concentrations of these drugs. Consequently, the dose of CY3A4 substrates should be administered with caution when given in combination with lopinavir/ritonavir. And the induction effects of ritonavir on enzymes (CYP 3A, 1A2, 2B6, 2C9, 2C19, and diphosphate-glucuronosyltransferase) may result in lowered plasma concentrations and decrease of efficacy of the co-administered medicinal products (respective substrate), or may increase the concentrations of the active or toxic metabolite (in the case of prodrugs) (Cao et al. 2020; Foisy et al. 2008; Kim 2002; Mansuri et al. 2020).
Table 3 summarizes the recommended uses of APs with lopinavir/ritonavir. There are no APs with little to no evidence of clinically significant interaction expected. We recommend olanzapine, with caution and monitoring patients for reduced clinical effect (ritonavir induction of CYP1A2), and risperidone, monitoring for increased AP effects and the risk of QT prolongation and/or TdP.
Information about risk ratings, severity, and patient management in each database is summarized in Supplement Table 4.
Drug-drug interaction between APs and remdesivir
Lexicomp® Drug Interactions and Liverpool© Drug Interaction Group report that there are no significant risks. Remdesivir is not included in the Micromedex® Solutions Drugs Interactions database.
Drug-drug interaction between APs and favipiravir
Favipiravir are not included in the Micromedex® Solutions Drugs Interactions database. Lexicomp® Drug Interactions reports no significant risks and Liverpool© Drug Interaction Group shows yellow risk with chlorpromazine and quetiapine. Favipiravir is metabolized by aldehyde oxidase and quetiapine and chlorpromazine are known to inhibit this enzyme in vitro. However, the clinical relevance of aldehyde oxidase inhibition remains to be established. The yellow zone recommendations reflect the most conservative approach.
Drug-drug interaction between APs and tocilizumab
Micromedex® Solutions Drug Interactions presents no drug-drug interaction, and although the Liverpool© Drug Interaction Group reports risk of hematological toxicity with clozapine, it does not present evidence of clinically significant risk with the rest of antipsychotics. However, according to Lexicomp® Drug Interactions, tocilizumab may decrease the serum concentration of CYP3A4 substrates like aripiprazole, brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine.
The information on risk rating, severity, and patient management for each database is summarized in Supplement Table 5.
The authors’ consensus recommendation (see Table 1) is that brexpiprazole, cariprazine, and lurasidone be used with caution. This is the most conservative approach, because they are not included in the Liverpool© database, and therefore, agreement is not possible.
Drug-drug interaction between APs and baricinitib
Baricinitib is not included in the Liverpool© Drug Interaction Group and Micromedex® Solutions Drugs Interactions shows no drug-drug interaction with antipsychotics. The summary information about the risk rating, severity, and patient management for each database is available in eTable 6 in the Supplement.
All antipsychotics are recommended, but not clozapine because of hematological risk (risk rating in zone yellow).
Drug-drug interaction between APs and anakinra
Anakinra is not included in the Liverpool© Drug Interaction Group. Micromedex® Solutions Drugs Interactions and Lexicomp® Drug Interactions do not report any drug-drug interaction with antipsychotics.
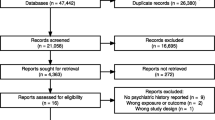
Results of the systematic review
Only 7 case reports out of the 391 studies screened and assessed for eligibility were finally included in the systematic revision, after applying inclusion and exclusion criteria (Supplement eFig. 1). Data from all included studies are summarized in Table 4. Two case reports are considered highly probable drug interaction according to DIPS, one with lopinavir/ritonavir and quetiapine (with priapism as the clinical outcome), and with ritonavir/indinavir and risperidone (with acute dystonia and tremor exacerbation as the clinical outcome). There were no deaths among the 8 patients, but two fatal outcomes, one patient in coma (Risperidone plus Ritonavir) and 2 others with neuroleptic malignant syndrome (ritonavir plus aripiprazole, and ritonavir plus risperidone); however, all of them remitted.
There was no study or case report about QT prolongation and/or TdP between the drugs of our study.
Discussion
According to the DDIs databases and our systematic review, remdesivir, baricinitib, and anakinra may be concomitantly used with antipsychotics by clinicians for managing delirium, agitation, or behavioral problems in COVID-19 patients with no risk of drug-drug interaction (except for hematological risk with clozapine and baricinitib reported only in one database). Favipiravir only needs caution with chlorpromazine and quetiapine (reported only in one database).
Tocilizumab is quite safe for use in combination with antipsychotics; however, care should be taken with aripiprazole, brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine, because of decreased exposure of APs, and with clozapine because of hematological risk. Tocilizumab binds to and inhibits the proinflammatory cytokine interleukin-6 (IL-6), and IL-6 decreased CYP3A4 mRNA by over 90% (Aitken and Morgan 2007). Tocilizumab may restore CYP3A4 activity and thus increase CYP3A4 substrate metabolism. This effect may persist for several weeks following discontinuation of therapy due to the long half-life of tocilizumab.
The COVID-19 treatments with the most risk for coadministration with antipsychotics are chloroquine, hydroxychloroquine, azithromycin, and lopinavir/ritonavir.
Most of the DDIs databases point out the increased risk of prolonged QT interval with coadministration of chloroquine, hydroxychloroquine, and azithromycin with antipsychotics. Nevertheless (White 2007), the cardiotoxicity of antimalarial drugs, with the exception of quinidine and halofantrine, which produce clinically significant prolongation of ventricular repolarization, remains to be confirmed (Haeusler et al. 2018; White 2007). In the present COVID scenario, Borba et al. (Borba et al. 2020) do not recommend the highest chloroquine dosage for patients critically ill with COVID-19 because of its potential safety hazards, especially when taken concurrently with azithromycin and oseltamivir. Risk of prolonged QTc (over 500 ms) was higher in patients receiving high doses (600 mg twice daily for 10 days) than a low-dosage group (450 mg twice on Day 1 followed by once daily for four days), 18.9% versus 11.1%, respectively. It is worth mentioning that all patients were also receiving azithromycin, and nearly all were receiving oseltamivir (both also prolong QT interval) (Fihn et al. 2020). Although treatment with hydroxychloroquine, azithromycin, or both was not compared with either treatment, in 1438 hospitalized patients with COVID-19, it was not significantly associated with differences in in-hospital mortality (Rosenberg et al. 2020). In addition, grading antipsychotics by torsadogenic risk is not a simple issue, and attributable risk varies depending on the source (Leucht et al. 2013; Raschi et al. 2013).
Our work also suggests that the coadministration of protease inhibitors, such as lopinavir/ritonavir, with APs should be cataloged as the most problematic combination, because of the CYP DDIs (Ernest et al. 2005) and the risk of QT prolongation and/or TdP. Coadministration of ritonavir and medicinal products primarily metabolized by CYP3A4 may result in increased plasma concentrations. Since ritonavir should be considered a pan-inducer of CYP450 (ritonavir induces cytochrome P450 enzymes 3A, 1A2, 2B6, 2C9, and 2C19, as well as glucuronyl transferase), the management of induction interactions is often unclear. When the substrate drug has a narrow therapeutic window or when the consequences of treatment failure are severe, alternative agents should be considered. Patients should be closely monitored for decreased efficacy of substrate medications when ritonavir is initiated. And, if ritonavir is stopped, lower doses of substrate medications should be considered and patients should be monitored very closely for toxicity. It is important to indicate that ritonavir has mixed effect, induction and inhibition on CYP3A (although inhibitory interactions are more prevalent); it has been proposed as an autoinducer (Hsu et al. 1997). The extent of induction was greater at higher doses, minimal al the lowest dose, and stabilized after 2 weeks. The simultaneous inhibition and induction effect requires further study with other CYP3A substrates, and caution in the study design (dose of ritonavir, duration of study, other co-administered protease inhibitor) when interpreting the results (Foisy et al. 2008). Clinicians should take this into account and be extremely cautious with patients.
Although none of the APs seems to be recommended for used in combination with lopinavir/ritonavir, olanzapine might be considered as the safest option according to DDIs databases and also because none of the case report describing DDI has been reported. Ritonavir decreases systemic exposure to olanzapine through the induction of both CYP1A2 and UGT. Thus, patients on concomitant olanzapine and ritonavir may require higher doses (up to 50% more) of olanzapine to achieve similar plasma concentrations (Meemken et al. 2015; Penzak et al. 2002).
Strengths and limitations
The use of drug interaction databases can be a useful clinical tool, but the lack of agreement between them limits their use (Abarca et al. 2004; Chao and Maibach 2005; Fulda et al. 2000; Monteith and Glenn 2019; Vitry 2007). Our study reviewed DDIs in three databases which fulfill quality criteria (Patel and Beckett 2016; Rodríguez-Terol et al. 2009), and a systematic review of the literature was also dome in search of possible clinical outcomes of these DDIs.
The main treatments used internationally for the treatment of COVID-19 were included, as well as most antipsychotics.
As limitations of our study, it should be noted that no studies were found beyond clinical cases which examined clinical results of the interaction between the drugs studied, so this information could not be presented. Another limitation to be acknowledged is the fact that ritonavir is CYP450 pan-inducer, and therefore, some inconsistencies between the interaction profiles described in the current datasets utilized in our manuscript and those described in particular scientific reports exploring the potential interaction between ritonavir and specific antipsychotics might emerge.
Conclusions
In conclusion, the growing use of antipsychotics in COVID-19 patients calls on the urges development evidence-based guidelines that can help clinicians decide on the safest treatment combination and the level of monitoring that would be warranted for each particular patient. Clinicians prescribing antipsychotics should be aware of the likely risk of drug-drug interaction with COVID-19 medication and may benefit from taking into account present recommendations of use to preserve patient safety. The main interactions between COVID-19 drugs and antipsychotics are the risk of QT prolongation and/or TdP, and CYP interactions.
References
Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van Bergen RC, Lipton RB (2004) Concordance of severity ratings provided in four drug interaction compendia. J Am Pharm Assoc 44(2):136–141. https://doi.org/10.1331/154434504773062582
Acciavatti T, Martinotti G, Corbo M, Cinosi E, Lupi M, Ricci F, Di Scala R, D’Ugo E, De Francesco V, De Caterina R, di Giannantonio M (2017) Psychotropic drugs and ventricular repolarisation: the effects on QT interval, T-peak to T-end interval and QT dispersion. J Psychopharmacol 31(4):453–460. https://doi.org/10.1177/0269881116684337
Aitken AE, Morgan ET (2007) Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos 35(9):1687–1693. https://doi.org/10.1124/dmd.107.015511
Aung GL, O’Brien JG, Tien PG, Kawamoto LS (2010) Increased aripiprazole concentrations in an HIV-positive male concurrently taking duloxetine, darunavir, and ritonavir. Ann Pharmacother 44(11):1850–1854. https://doi.org/10.1345/aph.1P139
Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A et al (2020) Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infection: a randomized clinical trial. JAMA Netw Open 3(4):e208857. https://doi.org/10.1001/jamanetworkopen.2020.8857
Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D (2020) Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. https://doi.org/10.1016/j.jinf.2020.04.017
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C et al (2020) A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2001282
Chao SD, Maibach HI (2005) Lack of drug interaction conformity in commonly used drug compendia for selected at-risk dermatologic drugs. Am J Clin Dermatol 6(2):105–111. https://doi.org/10.2165/00128071-200506020-00005
Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents:105932. https://doi.org/10.1016/j.ijantimicag.2020.105932
Conley RR, Kelly DL (2007) Drug-drug interactions associated with second-generation antipsychotics: considerations for clinicians and patients. Psychopharmacol Bull 40(1):77–97
Ely E, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard G, Inouye S (2001) The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 27(12):1892–1900. https://doi.org/10.1007/s00134-001-1132-2
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291(14):1753–1762. https://doi.org/10.1001/jama.291.14.1753
Ernest CS, Hall SD, Jones DR (2005) Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther 312(2):583–591. https://doi.org/10.1124/jpet.104.075416
Etchegoyen CV, Keller GA, Mrad S, Cheng S, Di Girolamo G (2017) Drug-induced QT interval prolongation in the intensive care unit. Curr Clin Pharmacol 12(4):210–222. https://doi.org/10.2174/1574884713666180223123947
Fihn SD, Perencevich E, Bradley SM (2020) Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA Netw Open 3(4):e209035–e209035. https://doi.org/10.1001/jamanetworkopen.2020.9035
Foisy MM, Yakiwchuk EM, Hughes CA (2008) Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother 42(7):1048–1059. https://doi.org/10.1345/aph.1K615
Fulda TR, Valuck RJ, Zanden JV, Parker S, Byrns PJ, Acknowledgments, U. P. D. U. R. A. P. of the U. P. D. U. R. A. P. are listed in the (2000) Disagreement among drug compendia on inclusion and ratings of drug-drug interactions. Curr Ther Res 8(61):540–548
Gareri P, De Fazio P, Manfredi VGL, De Sarro G (2014) Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. J Clin Psychopharmacol 34(1):109–123. https://doi.org/10.1097/JCP.0b013e3182a6096e
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain J-M, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents:105949. https://doi.org/10.1016/j.ijantimicag.2020.105949
Geraci MJ, McCoy SL, Crum PM, Patel RA (2010) Antipsychotic-induced priapism in an HIV patient: a cytochrome P450-mediated drug interaction. Int J Emerg Med 3(2):81–84. https://doi.org/10.1007/s12245-010-0175-y
Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ (2020) Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin Proc 0(0). https://doi.org/10.1016/j.mayocp.2020.03.024
Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D’Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW et al (2020) COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 116(10):1666–1687. https://doi.org/10.1093/cvr/cvaa106
Haeusler IL, Chan XHS, Guérin PJ, White NJ (2018) The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med 16(1):200. https://doi.org/10.1186/s12916-018-1188-2
Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F (2020) Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 0(0) null. https://doi.org/10.1056/NEJMc2008597
Horn JR, Hansten PD, Chan L-N (2007) Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother 41(4):674–680. https://doi.org/10.1345/aph.1H423
Hsu A, Granneman GR, Witt G, Locke C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Niu P, Decourt JP, Fourtillan JB, Girault J, Leonard JM (1997) Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother 41(5):898–905
Jean S-S, Lee P-I, Hsueh P-R (2020) Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect = Wei Mian Yu Gan Ran Za Zhi. https://doi.org/10.1016/j.jmii.2020.03.034
Jin B, Liu H (2019) Comparative efficacy and safety of therapy for the behavioral and psychological symptoms of dementia: a systemic review and Bayesian network meta-analysis. J Neurol 266(10):2363–2375. https://doi.org/10.1007/s00415-019-09200-8
Jover F, Cuadrado J-M, Andreu L, Merino J (2002) Reversible coma caused by risperidone-ritonavir interaction. Clin Neuropharmacol 25(5):251–253. https://doi.org/10.1097/00002826-200209000-00004
Kelly DV, Béïque LC, Bowmer MI (2002) Extrapyramidal symptoms with ritonavir/indinavir plus risperidone. Ann Pharmacother 36(5):827–830. https://doi.org/10.1345/aph.1A335
Kennedy WK, Jann MW, Kutscher EC (2013) Clinically significant drug interactions with atypical antipsychotics. CNS Drugs 27(12):1021–1048. https://doi.org/10.1007/s40263-013-0114-6
Kim RB (2002) Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev 34(1–2):47–54. https://doi.org/10.1081/dmr-120001389
King JR, Wynn H, Brundage R, Acosta EP (2004) Pharmacokinetic enhancement of protease inhibitor therapy. Clin Pharmacokinet 43(5):291–310. https://doi.org/10.2165/00003088-200443050-00003
Kotfis K, Marra A, Ely EW (2018) ICU delirium—a diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol Intensive Ther 50(2):160–167. https://doi.org/10.5603/AIT.a2018.0011
Lee SI, Klesmer J, Hirsch BE (2000) Neuroleptic malignant syndrome associated with use of risperidone, ritonavir, and indinavir: a case report. Psychosomatics 41(5):453–454. https://doi.org/10.1176/appi.psy.41.5.453
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet (London, England) 382(9896):951–962. https://doi.org/10.1016/S0140-6736(13)60733-3
Maher AR, Maglione M, Bagley S, Suttorp M, Hu J-H, Ewing B, Wang Z, Timmer M, Sultzer D, Shekelle PG (2011) Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 306(12):1359–1369. https://doi.org/10.1001/jama.2011.1360
Mansuri Z, Adnan M, Jolly T (2020) Ritonavir/lopinavir and its potential interactions with psychiatric medications: a COVID-19 perspective. Primary Care Companion CNS Disord 22(3):0–0. https://doi.org/10.4088/PCC.20com02677
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127
Meemken L, Hanhoff N, Tseng A, Christensen S, Gillessen A, Meemken L, Hanhoff N, Tseng A, Christensen S, Gillessen A (2015) Drug-drug interactions with antiviral agents in people who inject drugs requiring substitution therapy. Ann Pharmacother 49(7):796–807
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Monteagudo LA, Boothby A, Gertner E (2020) Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. https://doi.org/10.1002/acr2.11135
Monteith S, Glenn T (2019) A comparison of potential psychiatric drug interactions from six drug interaction database programs. Psychiatry Res 275:366–372. https://doi.org/10.1016/j.psychres.2019.03.041
National Institute for Health and Care Excellence (NICE) in collaboration with NHS England and NHS Improvement (2020) Managing COVID-19 symptoms (including at the end of life) in the community: summary of NICE guidelines. BMJ (Clinical Research Ed) 369:m1461. https://doi.org/10.1136/bmj.m1461
O’Hanlon S, Inouye SK (2020) Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing. https://doi.org/10.1093/ageing/afaa094
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5(1):210. https://doi.org/10.1186/s13643-016-0384-4
Patel RI, Beckett RD (2016) Evaluation of resources for analyzing drug interactions. J Med Lib Assoc 104(4):290–295. https://doi.org/10.3163/1536-5050.104.4.007
Penzak SR, Hon YY, Lawhorn WD, Shirley KL, Spratlin V, Jann MW (2002) Influence of ritonavir on olanzapine pharmacokinetics in healthy volunteers. J Clin Psychopharmacol 22(4):366–370. https://doi.org/10.1097/00004714-200208000-00006
Pollack TM, McCoy C, Stead W (2009) Clinically significant adverse events from a drug interaction between quetiapine and atazanavir-ritonavir in two patients. Pharmacotherapy 29(11):1386–1391. https://doi.org/10.1592/phco.29.11.1386
Raschi E, Poluzzi E, Godman B, Koci A, Moretti U, Kalaba M, Bennie M, Barbui C, Wettermark B, Sturkenboom M, De Ponti F (2013) Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe. PLoS One 8(11):e81208. https://doi.org/10.1371/journal.pone.0081208
Ray WA, Chung CP, Murray KT, Hall K, Stein CM (2009) Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 360(3):225–235. https://doi.org/10.1056/NEJMoa0806994
Rivière J, van der Mast RC, Vandenberghe J, Van Den Eede F (2019) Efficacy and tolerability of atypical antipsychotics in the treatment of delirium: a systematic review of the literature. Psychosomatics 60(1):18–26. https://doi.org/10.1016/j.psym.2018.05.011
Roden DM (2004) Drug-induced prolongation of the QT interval. N Engl J Med 350(10):1013–1022. https://doi.org/10.1056/NEJMra032426
Rodríguez-Terol A, Caraballo MO, Palma D, Santos-Ramos B, Molina T, Desongles T, Aguilar A (2009) Calidad estructural de las bases de datos de interacciones. Farm Hosp 33(3):134–146. https://doi.org/10.1016/S1130-6343(09)71155-9
Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, Weinberg P, Kirkwood J, Muse A, DeHovitz J, Blog DS, Hutton B, Holtgrave DR, Zucker HA (2020) Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. https://doi.org/10.1001/jama.2020.8630
Salvo F, Pariente A, Shakir S, Robinson P, Arnaud M, Thomas S, Raschi E, Fourrier-Réglat A, Moore N, Sturkenboom M, Hazell On Behalf Of Investigators Of The Aritmo Consortium, L., & Investigators of the ARITMO Consortium (2016) Sudden cardiac and sudden unexpected death related to antipsychotics: a meta-analysis of observational studies. Clin Pharmacol Ther 99(3):306–314. https://doi.org/10.1002/cpt.250
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. https://doi.org/10.1001/jama.2020.6019
Shukla AM, Archibald LK, Shukla AW, Mehta HJ, Cherabuddi K (2020) Chloroquine and hydroxychloroquine in the context of COVID-19. Drugs Context 9. https://doi.org/10.7573/dic.2020-4-5
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Testai L, Bianucci AM, Massarelli I, Breschi MC, Martinotti E, Calderone V (2004) Torsadogenic cardiotoxicity of antipsychotic drugs: a structural feature, potentially involved in the interaction with cardiac HERG potassium channels. Curr Med Chem 11(20):2691–2706. https://doi.org/10.2174/0929867043364351
Truven Health Analytics. Micromedex® Solutions Drugs Interaction [electronic version] (2020) Retrieved from http://www.micromedexsolutions.com. Accessed 23 June 2020
University of Liverpool. Liverpool COVID-19 Interactions (2020) Retrieved from https://www.covid19-druginteractions.org/prescribing-resources. Accessed 23 June 2020
van der Linde RM, Dening T, Matthews FE, Brayne C (2014) Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 29(6):562–568. https://doi.org/10.1002/gps.4037
Vasilevskis EE, Chandrasekhar R, Holtze CH, Graves J, Speroff T, Girard TD, Patel MB, Hughes CG, Cao A, Pandharipande PP, Ely EW (2018) The cost of ICU delirium and coma in the intensive care unit patient. Med Care 56(10):890–897. https://doi.org/10.1097/MLR.0000000000000975
Vieweg WVR, Wood MA, Fernandez A, Beatty-Brooks M, Hasnain M, Pandurangi AK (2009) Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging 26(12):997–1012. https://doi.org/10.2165/11318880-000000000-00000
Vitry AI (2007) Comparative assessment of four drug interaction compendia. Br J Clin Pharmacol 63(6):709–714. https://doi.org/10.1111/j.1365-2125.2006.02809.x
Wellens HJJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kääb S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA, Wilde AA (2014) Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 35(25):1642–1651. https://doi.org/10.1093/eurheartj/ehu176
Whiskey E, O’Flynn D, Taylor D (2018) Clozapine, HIV and neutropenia: a case report. Ther Adv Psychopharmacol 8(12):365–369. BASE. https://doi.org/10.1177/2045125318804499
White NJ (2007) Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 7(8):549–558. https://doi.org/10.1016/S1473-3099(07)70187-1
Wolters Kluwer (2020) Wolters Kluwer. Lexicomp® Interactions Module [Internet]. Retrieved from https://www.wolterskluwercdi.com/lexicomp-online/. Accessed 23 June 2020
Woosley RL, Heise CW, Gallo T, Woosley RD, and Romero KA, QTFactors List (n.d.-a) Retrieved from https://www.crediblemeds.org/ndfa-list/. Accessed 26 Sep 2020
Woosley RL, Heise CW, Romero KA CredibleMeds ® (2020) Retrieved from https://www.crediblemeds.org/. Accessed 23 June 2020
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–141. https://doi.org/10.1016/j.pharmthera.2012.12.007
Acknowledgments
We would like to thank Carmen Jimenez, who encouraged us to start this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Plasencia-García B.O. has received honoraria for consulting/advisory boards from Otsuka Pharmaceuticals, Lundbeck, and Janssen Johnson & Johnson and lecture honoraria from Otsuka Pharmaceuticals, Lundbeck, Janssen Johnson & Johnson, Angelini, and Pfizer. Crespo-Facorro B. has received honoraria for consulting/advisory boards from Otsuka Pharmaceuticals, Takeda, and Angelini and lecture honoraria from Janssen Johnson & Johnson, Lundbeck, Roche, and Otsuka Pharmaceutical. The other authors declare that they have no known conflicts of interest. No pharmaceutical industry or institutional sponsors participated in the study design, data collection, analysis, and interpretation of the results
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 80 kb)
Rights and permissions
About this article
Cite this article
Plasencia-García, B.O., Rodríguez-Menéndez, G., Rico-Rangel, M.I. et al. Drug-drug interactions between COVID-19 treatments and antipsychotics drugs: integrated evidence from 4 databases and a systematic review. Psychopharmacology 238, 329–340 (2021). https://doi.org/10.1007/s00213-020-05716-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05716-4