Abstract
Rationale
Problematic patterns of gambling are characterised by loss of control and persistent gambling often to recover losses. However, little is known about the mechanisms that mediate initial choices to begin gambling and then continue to gamble in the face of losing outcomes.
Objectives
These experiments first assessed gambling and loss-chasing performance under different win/lose probabilities in C57Bl/6 mice, and then investigated the effects of antagonism of 5-HT2CR with SB242084, 5-HT1AR agonism with 8-OH-DPAT and modafinil, a putative cognitive enhancer.
Results
As seen in humans and other species, mice demonstrated the expected patterns of behaviour as the odds for winning were altered increasing gambling and loss-chasing when winning was more likely. SB242084 decreased the likelihood to initially gamble, but had no effects on subsequent gambling choices in the face of repeated losses. In contrast, 8-OH-DPAT had no effects on choosing to gamble in the first place, but once started 8-OH-DPAT increased gambling choices in a dose-sensitive manner. Modafinil effects were different to the serotonergic drugs in both decreasing the propensity to initiate gambling and chase losses.
Conclusions
We present evidence for dissociable effects of systemic drug administration on different aspects of gambling behaviour. These data extend and reinforce the importance of serotonergic mechanisms in mediating discrete components of gambling behaviour. They further demonstrate the ability of modafinil to reduce gambling behaviour. Our work using a novel mouse paradigm may be of utility in modelling the complex psychological and neurobiological underpinnings of gambling problems, including the analysis of genetic and environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increased access to opportunities to gamble in society, on the high street or via social media, more people are gambling these days than ever before. For most people, gambling is something that they can dip into occasionally and easily control, but for others, gambling can become a major problem affecting work and home life, personal relationships and financial security (Gainsbury 2015). Efforts to understand pathological gambling reveal a complex mix of genetic, familial and environmental risk factors, the former including gene variants that influence monoaminergic systems (Hodgins et al. 2011) and the latter socio-economic status, education, gender and accessibility to gambling platforms (van den Bos et al. 2013; Gainsbury 2015). Gambling problems are mediated by altered neuromodulation within mesolimbic reinforcement sites and altered neural responses to monetary rewards and gambling-related cues (Zack and Poulos 2004; Chase and Clark 2010; Balodis et al. 2012; Worhunsky et al. 2014). These dysfunctional brain responses may mediate the altered cognitions that promote gambling and, in vulnerable individuals, gambling problems (Campbell-Meiklejohn et al. 2008; Clark et al. 2009; Clark 2010). Current treatments include cognitive and/or behavioural therapies (Stea and Hodgins 2011) but also a variety of pharmacological options including SSRIs, mood stabilisers, atypical anti-psychotics and opioid antagonists (Grant et al. 2014). These therapies have uncertain efficacy, especially over the longer term, and there remains a need to better understand the neurobiology and risk factors associated with gambling behaviours and problematic gambling in order to identify more effective interventions and therapeutic targets (Potenza et al. 2013; Gainsbury 2015).
The progression from recreational gambling to pathological gambling is thought to be due to the loss of control and development of the characteristic loss-chasing behaviour (Lesieur 1979; Breen and Zuckerman 1999). Loss-chasing is the tendency to continue gambling or escalate stakes in the face of successive and accumulating losses (mainly financial) and may occur within a single session or over the longer term—both with the ultimate aim to ‘get even’ (Lesieur 1977, 1979). Between-session loss-chasing occurs, as the name suggests, over a longer period of time and may include multiple gambling sessions (Lesieur 1977). The majority of research has investigated within-session loss-chasing, where an individual attempts to recoup losses within a single session (Parke et al. 2016). Behavioural economic perspectives attribute loss-chasing to a convex function linking accumulating monetary losses to only diminishing marginal reductions in subjective value or utility, promoting further risk-seeking choices (Kahneman and Tversky 1979; Kahneman and Tversky 2000). However, in clinical populations, loss-chasing is markedly exaggerated and is associated with heightened trait impulsivity and erroneous false beliefs that support continued play (Breen and Zuckerman 1999). Loss-chasing is also associated with alcohol use and its disinhibitory effects (O'Connor and Dickerson 2003) and can be increased by alcohol consumption (Kyngdon and Dickerson 1999). These behavioural features make loss of control and consequent loss-chasing a key mechanism by which problem gambling produces its adverse financial, occupational and social consequences (Lesieur 1979).
There have been limited previous studies of the pharmacological basis of within-session loss-chasing. Using an experimental model in which human volunteers chose between gambling to recover accumulating (notional) losses by doubling their stakes or ‘quitting the chase’, Campbell-Meiklejohn and colleagues (Campbell-Meiklejohn et al. 2008, 2011) demonstrated that loss-chasing involves dissociable roles for monoamine systems, with serotonin activity modulating the persistence of chasing behaviour, but dopamine and, in particular, D2/D3 receptor (R) activity modulating the evaluation of losses worth chasing (Campbell-Meiklejohn et al. 2011, 2012; Rogers 2011). Exploiting the foraging preference of animals to access food promptly (Bateson and Kacelnik 1995; Kacelnik and Bateson 1996), Rogers et al. (2013) using rats modelled within-session loss-chasing as choices between immediate food access versus delayed access to food rewards (timeouts) over successive bad choice outcomes. With accumulating increases in delay to receiving reward as the penalty for continued gambling (and losing), whereas as quitting gambling would bring about reward sooner. Consistent with the human data (Campbell-Meiklejohn et al. 2011, 2012), the 5-HT1AR agonist 8-OH-DPAT and D2R antagonist eticlopride, diminished loss-chasing behaviour, increasing quitting at the first opportunity, whilst the D1R antagonist, SCH23390, had little effect (Rogers et al. 2013).
Both behavioural economic arguments and also the possibility that loss-chasing may be supported by behavioural momentum effects (defined as the persistence of gambling choices on the basis of previous winning outcomes/rewards in the same context) raise the possibility that different mechanisms mediate the commencement of gamble choices and their persistence. One hypothesis here, maybe the allocation of behavioural control of reinforcement learning balanced between model-based and mode-free systems, the former centred in prefrontal cortical regions and the latter in striatal regions of the brain. Complex or demanding situations may transition processing from the inflexible model-free system to the more flexible goal-directed model-based processing system (Daw et al. 2005; Russek et al. 2017; Kim et al. 2019). In terms of gambling and loss-chasing, then these systems could relate to the habitual nature of gambling (model-free), and the goal-directed actions underlying loss-chasing (model-based). Here, we investigate this dissociation using an adapted model of within-session loss-chasing for mice and use specific drug challenges to demonstrate novel pharmacological dissociations between the initial decision to gamble (to avoid timeouts and gain immediate palatable condensed milk rewards) and successive choices over pre-programmed sequences of losing outcomes.
Our data provide evidence consistent with the existence of neurobiologically distinct mechanisms underlying gambling and loss-chasing behaviour based on serotonergic signalling, via 5-HT2CR and 5-HT1AR mechanisms and also, for the first time in an animal model, the administration of the putative ‘cognition enhancer’ modafinil, which may have therapeutic potential in pathological gambling (Łabuzek et al. 2014; Pettorruso et al. 2014). In using mice in these studies, we also further demonstrate the tractability of this species in investigating complex behaviour and provide a new platform that can be used for the pharmacological or genetic investigation of gambling behaviours.
Methods and materials
Subjects
Two cohorts of male C57Bl/6OlaHsd mice (Envigo, UK) (N = 20/cohort), 3 months old at the start of the experiment, were housed in groups of four, in a vivarium (temperature: 21 ± 2 °C, humidity 50% ± 10) and a 12:12 light/dark cycle (lights on at 07:00 h). Food was available ad libitum; following 2 weeks of habituation and handling, the mice were placed on a home cage water restriction schedule of 2 h access/day, maintained for the duration of the experiment to motivate the animals to work in the task. Animals were treated in accordance with the Animal (Scientific Procedures) Act (United Kingdom, 1986).
Apparatus
The gambling/loss-chasing task (G/LCT) was performed in four mouse touchscreen chambers (Campden Cognition, UK, and see Bussey et al. 2012), under the control of custom written software (ABET, Campden Cognition, UK). Touchscreens were occluded by black Perspex masks with three 70 mm square response apertures 20 mm from the grid base of the chamber and positioned equally across the width of the mask. In the opposite side of the chamber, 5 mm from the grid base, was a 20 mm square recess (25 mm deep) from which liquid reward was delivered. Infra-red beams were used to record motor activity.
Procedure
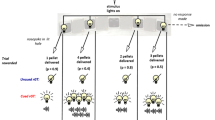
Following 2 weeks home cage water restriction schedule, the mice were habituated to the liquid reward of 10% condensed milk (Nestle Ltd., UK) solution (Humby et al. 1999, 2005). Subjects were habituated to the touchscreen chambers for 3 days and then trained to make a response to the central touchscreen stimulus location to earn reward (see Supplementary Methods Figure 1. When mice showed stable responding (> 40 responses/session for two consecutive sessions), the G/LCT was commenced with an initial training baseline version of the task in which touch responses to the gamble stimulus produced winning and losing outcomes with equal probabilities, following which the full G/LCT schedule was implemented which included loss-chasing options (see Fig. 1). All sessions in the touchscreen chambers were performed in darkness.
Schematic of the touchscreen gambling/loss-chasing task (G/LCT). The procedure was adapted from Rogers et al. (2013) and extended to a touchscreen platform in mice. A key modification to the current task was the introduction of procedures allowing a more systematic evaluation of loss-chasing behaviour over repeated programmed losses, allowing the opportunity for comparisons of loss-chasing to other components of gambling behaviour, such as the initial decision to quit or gamble. See main text and Supplementary Methods Figure 2 for further details.
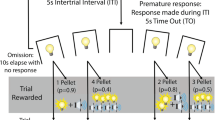
The procedure for the G/LCT, adapted from Rogers et al. (2013), was modified for a touchscreen platform. Two basic types of trial were presented equally and randomly through each session: ‘no-choice’ and ‘choice’ (Fig. 1). In a no-choice trial, which was designed to maintain a high level of responding, the initiation stimulus was presented to the central aperture of the touchscreen, touching the stimulus resulted in a delivery of 22 μl of condensed milk reward in the food magazine. The other 50% of trials were choice trials in which ‘quit’ and ‘gamble’ choice stimuli (‘fan’ and ‘marbles’ patterns, respectively, see Fig. 1) were presented in the left and right apertures, following a response to the central initiation stimulus, with the location randomly assigned for each trial. Choosing the quit stimulus resulted in no reward and a time-out period of 8 s signalled by illumination of the houselight, following which a new trial of either no-choice or choice was initiated. Thus, quitting this initial choice prevented the gambling option and had the potential to bring about a new trial more quickly. In contrast to a quit response, selecting the gamble stimulus generated, pseudo-randomly, a reward delivery of 60 μl reward (a ‘win’) or commenced a time-out period of 8 s, signalled by illumination of the houselight (a ‘loss’). In a standard gamble session, the ratio of wins to losses for the initial decision to gamble was set at 1:1, but this initial gambling choice was also assessed in sessions with different win/lose ratios (see below).
Collection of the reward after a winning outcome started a new no-choice or choice trial, but in the case of a losing outcome, and following the time-out as above, loss-chasing was assessed by continuing the current trial and offering the opportunity for further gamble or quit choices. Here, the animals were exposed to a series of programmed series of 2, 4 or 8 or losing outcomes (presented in pseudorandom order) that were terminated, should an animal continue to gamble and lose for the entire loss-chasing sequence available, by a winning outcome (see Supplementary Methods Figure 2). This possibility was included in the task design to encourage loss-chasing behaviour in the mice. At any time during a loss-chase sequence, the mouse could make a quit response and terminate these losing runs, receiving a time-out prior to the start of a new trial. In every trial, the stimuli would remain on the screen until a response had been made and thus, there were no omitted trials or choices. Thus, the penalty for gambling and losing (rather than quitting a trial) was to increase the delay to the next available reward (like in the Rogers et al. 2013 task design). A session was terminated once the subject had completed 60 trials or 20 min had elapsed.
Task manipulations
Initial decision to gamble
To test the extent to which the initial decision to gamble was sensitive to the probability of winning outcomes, the mice were tested with counter-balanced sequences of sessions with win/lose ratios of 1:4, and 4:1 for the initial gamble. Mice underwent a sequence of 6 consecutive sessions at each ratio, with a block of 1:1 win/lose ratio baseline sessions in between each manipulation.
Loss-chasing
The extent to which the various win/lose ratios pertaining for the initial gamble influenced the degree of subsequent loss-chasing behaviour was also assessed.
Pharmacological manipulations
The sensitivity of initial gambling choice and loss-chasing behaviours to pharmacological challenge were assessed in baseline 1:1 win/lose ratio sessions. This win/lose ratio was selected as it was equivalent to the rat task on which this study was based (Rogers et al. 2013), but also as it permitted both increases and decreases in gambling choices allowing for a non-biased hypothesis for the possible effects of the different drugs used. 5-HT2CR and 5-HT1AR were manipulated using SB242084 HCl (0, 0.1, 1, 5 mg/kg) and 8-OH-DPAT HCl (0, 0.03, 0.06, 0.1 mg/kg) (Cohort 1, N = 20 mice), respectively. Each of these drugs showing high selectivity for their target receptors (pKi = 9.0 and, pKi = 8.8, for SB242084 and 8-OH-DPAT, respectively (Kennett et al. 1997; Assié and Koek 2000). The effects of modafinil (0, 32, 64 mg/kg) were assessed in a separate group of mice (Cohort 2, N = 20) (all Tocris, UK). Doses of SB242084 (s.c., administered immediately prior to test), 8-OH-DPAT (i.p., administered 20 min prior to test) and modafinil (i.p., 30 min before test) were based on previous studies (Humby et al. 2013; Fernandes et al. 2013; Rogers et al. 2013). Drugs were prepared fresh in physiological saline each day. Each treatment was given using a Latin-square design, with at least 4 days between each dose; mice were not tested between the drug administration sessions.
Statistics
Main measures for the G/LCT were the % initial gambling choice, mean length of a loss-chase sequence, latency to make an initial gambling choice, proportion loss-chases ended by a quit response, number of trials started, latency to initiate a trial, latency to collect the reward and the numbers of food magazine entries, beam-breaks and touches to empty apertures of the touchscreen (blank touches). Scores calculated as percentages were arcsine transformed, and latencies were square root transformed prior to analysis. Data were analysed using SPSS (V.20, IBM Inc., USA) and were first assessed for normality, and then subjected to t test or ANOVA, if appropriate or equivalent non-parametric analyses. The comparison of performance with high (4:1) or low (1:4) win/lose ratios was analysed by paired two-tailed t tests or Wilcoxon signed-rank test. These analyses were performed with the two mouse cohorts combined, but also for comparisons between the two groups (Supplementary Results Figures 4 and 5). The effects of SB242084, 8-OH-DPAT and modafinil were analysed by separate within-subject ANOVAs or Friedman tests with a factor of DOSE (vehicle, 0.1, 1, 5 mg/kg, vehicle, 0.03, 0.06, 0.1 mg/kg, and vehicle, 32 and 64 mg/kg, for each drug, respectively). If significant, then post hoc pairwise comparisons were performed using Bonferroni or Wilcoxon signed-rank test, respectively, and adjusted for multiple comparisons. Criterion level of significance was set at the 0.05 level. All data are shown as mean ± standard error of the mean (S.E.M.).
Results
Prior to testing, all 40 mice in the experiment demonstrated significant preference for the condensed milk reward over water (c. 80% preference, see Supplementary Results Figure 1), consistent with previous groups of mice (Humby et al. 1999, 2005, 2013). Following habituation and shaping (see Supplementary Results Figure 2), the mice achieved reliable and stable performance in the G/LCT within 20 sessions (mean 19.73 ± 0.47 sessions).
C57Bl/6 mice will gamble and chase losses for food reward
Having learned to touch the screen for reward and shown consistent and stable performance in the baseline version of the G/LCT with 1:1 win/lose ratio, the mice were moved on to the task configuration that included a loss-chasing component. Under the baseline 1:1 win/lose ratio, for the initial decision to gamble or quit the animals made more gamble than quit choices (c.70% gamble choice, see Fig. 2a). As anticipated, the initial decision to gamble was also sensitive to the probability of gaining reward with more gamble selections (Fig. 2a, t39 = 5.57, p = 0.001, d = 0.33) and quicker responses (Fig. 2b, Z39 = 5.26, p = 0.001, d = 0.36) in sessions with high win/lose ratios than low win/lose ratios. These mice also demonstrated loss-chasing behaviour whereby following the initial choice to gamble and a loss the animals continued to gamble for reward despite suffering repeated further losses and associated time-out penalties. As shown in Fig. 2c, the mean length of consecutive loss-chases before quitting, under the baseline 1:1 win/lose ratio condition, was ~ 4 losses (the mice rarely persisted in chasing all 8 losses available, see Supplementary Results Figure 3a). Loss-chasing behaviour was sensitive to the prevailing win/lose ratio of the initial gamble with animals engaging in longer chases in sessions with a high win/lose ratio than in sessions with a low win/lose ratio (Fig. 2c, Z39 = 2.81, p = 0.005, d = 0.28). As two cohorts of mice were used in these studies, we have also performed between-cohort comparisons on these data, which demonstrated non-significant results (p > 0.05 for all analyses (Supplementary Results Figure 4). We also assessed the comparison between cohorts using a Bayesian approach (JASP V0.11, Netherlands) which demonstrated very weak effects for differences between the two cohorts of mice across all the measures (BF10 < 1, odds ratio relative to the null hypothesis). Therefore, both groups of mice showed equivalent responses as the win/lose ratio was altered. However, we did notice that there were some changes in performance with the baseline 1:1 win/lose ratio between the first time of test and on completion of the win/lose ratio assessments (Supplementary Results Figure 5). We assessed these differences and found that the propensity to gamble had significantly increased, and the loss-chase sequence length reduced for both cohorts of mice; however, we also found that these differences remained constant and unchanging through evaluation of the effects of the drug challenges (Supplementary Results Figure 5, comparison with vehicle treatment).
Effects of altering the odds of winning on initial gambling choice and loss-chasing behaviour in adult C57Bl/6 mice. When the odds of winning were high, the mice showed a greater initial preference to gamble (a), were quicker at making this choice (b), and were more likely to loss-chase (c). Data from the final day of testing at each schedule were used in the analysis. Data from sessions with a 1:1 win/lose ratio are shown for illustrative purposes and were not included in the statistical analysis. Data shows mean ± SE, for both mouse Cohorts combined (N = 40). ** denotes p < 0.01 and *** denotes p < 0.001 for the comparison between 4:1 and 1:4 win/ratio tests
5-HT2CR antagonism with SB242084 reduces the propensity to gamble without influencing loss-chasing
The effects of the 5-HT2CR antagonist SB242084 on the initial decision to gamble and subsequently chase losses are shown in Fig. 3. The effects of the drug showed dissociations between these different phases of behaviour. SB242084 at doses of 0.1, 1 and 5 mg/kg reduced the propensity to make an initial gamble; that is, the animals showed a clear reduction in gambling responses and, therefore, a corresponding increase in quitting, under drug (Fig. 3a, main effect of DOSE, F3,57 = 9.27, p = 0.001, η2 = 0.99). The effects of SB242084 on whether to gamble or quit occurred in the absence of effects on the speed of responding (Fig. 3b, main effect of DOSE, F3,57 = 2.24, p = 0.09, η2 = 0.54). The effects of SB242084 on the initial decision to gamble did not extend to subsequent loss-chasing, where behaviour was insensitive to drug, insofar as there were no effects at any dose on the persistence of gambling in the face of losing runs (Fig. 3c, main effect of DOSE, F3,57 = 0.29, p = 0.82, η2 = 0.10). The dissociations in behaviour were highly specific and occurred in the absence of drug-induced changes in general components of behaviour for example, the number of responses/session, latency to start a trial, locomotor activity and food magazine entries, although there was a tendency for an increased reward collection latency with the 5 mg/kg dose (Supplementary Results Figure 3).
Effects of 5-HT2CR antagonist SB242084 on initial gambling choice and loss-chasing behaviour in adult C57Bl/6 mice. At all doses used, SB242084 reduced the initial choice to gamble (a). There were no effects of SB242084 at any dose on the time taken to make the initial choice to gamble (b), or on the number of loss-chases made by the mice (c). For each of these sessions, the win/lose ratio was 1:1. Data shows mean ± SE, mice from Cohort 1 only (N = 20). *** denotes p < 0.001 for the comparison with vehicle treatment
5-HT1AR agonism with 8-OH-DPAT has no effects on the initial decision to gamble but increases loss-chasing
In contrast to SB242084, 8-OH-DPAT had no statistically significant effects on the initial choice to gamble (Fig. 4a). Note that the main effect of DOSE was close to significance (F3,57 = 2.86, p = 0.06, η2 = 0.58), because of elevated rates of gamble responses at the highest dose used, of 0.1 mg/kg; however, this dose was also associated with major disruptions in general components of behaviour, including marked slowing of choice responding (Fig. 4b, main effect of DOSE, F3,57 = 11.20, p = 0.001, η2 = 0.99) and a decline in the overall number of trials started in a session from ~ 60 under vehicle conditions to ~ 40 (see Supplementary Results Figure 4). There was evidence of specific effects of 8-OH-DPAT on loss-chasing behaviour. These effects were manifest as an increase in consecutive loss-chases at the lowest, 0.03 mg/kg, dose of drug (Fig. 4c, main effect of DOSE, F3,57 = 4.48, p = 0.01, η2 = 0.86, confirmed by post hoc comparison: p < 0.05 for 0.03 mg/kg dose to all other doses) in the absence of generalised effects on other behavioural measures (Supplementary Results Figure 4).
Effects of the 5-HT1AR agonist 8-OH-DPAT on initial gambling and loss-chasing behaviour in adult C57Bl/6Ola mice. 8-OH-DPAT did not significantly affect the initial choice of the mice to gamble (a), but increased the time taken to make this choice, although this was only at the 0.1 mg/kg dose used (b). Loss-chasing was influenced by 8-OH-DPAT, with a significant increase from vehicle that was specific to the lowest 0.03 mg/kg dose of the drug (c). For each of these sessions, the win/lose ratio was 1:1. Data shows mean ± SE, mice from Cohort 1 only (N = 20). * denotes p < 0.05 and ** p < 0.01 for comparison with vehicle treatment, respectively
Modafinil reduces both the propensity to gamble and loss-chasing behaviour
Modafinil reduced initial choices to gamble, thereby increasing quitting behaviour (Fig. 5a, main effect of DOSE, F2,38 = 4.28, p = 0.02, η2 = 0.18); post hoc comparisons showing that choices to start gambling at the 32 mg/kg dose were significantly diminished compared to vehicle (p = 0.035), but not with the 64 mg/kg dose (p = 0.84). The effects of the 32 mg/kg dose of modafinil in reducing gambling choices were not associated with any marked effects on the speed of responding; however, the 64 mg/kg dose reduced speed of responding (Fig. 5b, main effect of DOSE, F2,38 = 4.63, p = 0.02, η2 = 0.18; comparisons of vehicle and 32 mg/kg: p = 0.80, and vehicle and 64 mg/kg p = 0.045). Modafinil reduced the extent of subsequent loss-chasing but again only at the lower 32 mg/kg dose (Fig. 5c, main effect of DOSE, F2,38 = 7.14, p = 0.002, η2 = 0.27, post hoc comparisons of 32 mg/kg dose with vehicle and 64 mg/kg of p = 0.03 and p = 0.001, respectively). Neither dose was associated with any marked change in general features of behaviour save a slight tendency for the overall number of trials started to increase at the highest dose (Supplementary Results Figure 5).
Effects of modafinil on gambling and loss-chasing behaviour in adult C57Bl/6 mice. Modafinil reduced the initial decision to gamble by the mice at the 32 mg/kg dose with no significant effects of the higher 64 mg/kg dose (a). Modafinil reduced the time taken to make the decision to gamble, effects that reached significance at the 64 mg/kg dose but not 32 mk/kg dose (b). Loss-chasing was also influenced by modafinil, with a significant reduction in behaviour from vehicle at 32 mg/kg but not 64 mg/kg (c). For each of these sessions, the win/lose ratio was 1:1. Data shows mean ± SE, mice from Cohort 2 (N = 20). * denotes p < 0.05 for the comparison with vehicle treatment
Discussion
We have used a novel touchscreen-based paradigm in mice to investigate gambling behaviours. We established that hungry mice will gamble and loss-chase to achieve food reward, with the possible penalty of losing as increased delay to the next available reward. The mice were sensitive to the relative probabilities of winning/losing outcomes, as seen in humans and other species mice, by increased gambling and loss-chasing when winning was more likely or quitting gambling when winning was less likely. We found evidence for the existence of dissociable effects of serotonergic drugs and modafinil on different aspects of gambling behaviour. Antagonism of 5-HT2CR with SB242084, whilst decreasing the likelihood of C57Bl/6 mice to make an initial gambling choice, had no effects on the persistence of subsequent loss-chasing behaviours. In contrast, 5-HT1AR agonism, with low dose 8-OH-DPAT, had no effects on the likelihood of subjects choosing to initiate gambling, but once they had gambled tended to increase the subsequent loss-chasing behaviours. Modafinil at a dose of 32 mg/kg decreased both the propensity to engage in gambling and reduced loss-chasing. Our data are consistent with the suggestion that serotonin receptor systems may play dissociable roles in starting and then sustaining gambling responses in the face of continued losing outcomes (Campbell-Meiklejohn et al. 2011; Rogers et al. 2013); additionally, they indicate the efficacy of modafinil, a putative cognitive enhancer, to modify gambling behaviours.
Human studies have attempted to parse components of gambling behaviours and there is evidence for the existence of dissociable neural systems and pharmacological substrates underlying, for example, the decision to chase losses or quit (Campbell-Meiklejohn et al. 2008, 2011 and 2012). However, to our knowledge, there have been no explicit comparisons of the pharmacological mechanisms of initial decisions to gamble/quit and subsequent loss-chasing behaviour in either un-drugged or drug treated subjects. Behavioural economic perspectives, such as Prospect Theory (Kahneman and Tversky 1979; Kahneman and Tversky 2000), posit that within-session loss-chasing behaviour persists because in the case of typical human gambling behaviour, accumulating monetary losses generate only diminishing marginal reductions in subjective value (relative to gamblers’ current purse), prompting the persistence of risk-seeking choices (Campbell-Meiklejohn et al. 2008). According to this view, initial decisions to gamble are distinguished by the potential for greater reductions in subjective value compared to subsequent decisions to continue gambling. Additionally, loss-chasing may also be contributed to by a form of behavioural momentum, manifest as the persistence of choices previously but not currently rewarded in the same context (Kahneman and Tversky 1979; Nevin 2002). Irrespective of these theoretical speculations regarding the psychological underpinnings of gambling behaviours, the pharmacological data presented here suggest that 5-HT2CR and 5-HT1AR activity modulate risky choices to varying extents between the initial decision and subsequent loss-chasing.
Our findings add to the increasing evidence for serotonergic neurotransmitter influences on gambling behaviours as demonstrated in both human and animal model studies (Zeeb et al. 2009; Campbell-Meiklejohn et al. 2011; Rogers et al. 2013). A novel finding was the efficacy of SB242084 to alter gambling behaviour by reducing the propensity to make an initial gamble (increased quitting) with no effects on loss-chasing. Quitting the initial gambling choice, and receiving a time-out, could be interpreted as choosing the safer option which was more guaranteed to provide reward quicker than electing to gamble which induced further delays. Changes in 5-HT2CR function, specifically hypersensitivity, have been suggested to occur in pathological gamblers (Conversano et al. 2012). Hence, the data with SB242084 are consistent with human work; however, the precise psychological and brain mechanisms by which 5-HT2CR antagonism impacts on dissociable aspects of gambling behaviour are unknown but could be centred in the prefrontal cortex where these receptors are expressed (Pompeiano et al. 1994). One possible, hypothesis may be the involvement of cortical 5-HT2CR in top down processing and control of model-based and striatal model-free mechanisms for behavioural choice (Daw et al. 2005; Russek et al. 2017; Kim et al. 2019). In the current task, 5-HT2CR antagonism could influence the dissociation between these systems, via modulating striatal dopamine (Seymour et al. 2012) in the drive to move from initially gambling (and losing) to loss-chasing to pursue a potential win. However, previous studies have demonstrated the effects of SB242084 on response control and selection are complex, with evidence for both decreased (Humby et al. 2013) and increased impulsivity in control of choices (Winstanley et al. 2004; Fletcher et al. 2007; Robinson et al. 2008; Paterson et al. 2012). Acute activation of 5-HT2CR inhibits feeding (Clifton and Kennett 2006), and various 5-HT2CR agonists are currently registered to treat obesity (Palacios et al. 2017). SB242084 will reduce the anorectic effects of d-fenfluramine, mCPP or novelty-induced hypophagia, but does not affect food intake when administered alone (Vickers et al. 2001; Hewitt et al. 2002; Nahata et al. 2013). Thus, the findings observed here are unlikely to be a due to effects of SB242084 on feeding mechanisms or drive to achieve reward. Nonetheless, 5-HT2CRs are expressed in brain circuitries with established roles in mediating response control and responses to reward (Barnes and Sharp 1999; Chagraoui et al. 2016) and further investigation of these psychological functions in the context of explaining the putative specificity of 5-HT2CR blockade on gambling behaviours is warranted.
In previous work using rat models, 8-OH-DPAT has been shown to reduce loss-chasing behaviour (Rogers et al. 2013). Using the same dose range in the current mouse task, we observed no significant effects on the initial decision to gamble but instead an increase in loss-chasing that was confined to the lowest 0.03 mg/kg dose consistent with a pre-synaptic effect of 5-HT1AR agonism (i.e. reduced 5-HT release, Sharp et al. 1989). There are several possible reasons for the contrasting effects of 8-OH-DPAT on loss-chasing seen here and in Rogers et al. (2013) work, which may include species differences but also a task difference which may be interacting with reduced 5-HT release. Specifically, in the present study, the subjects could endure up to 8 successive losses with the possibility of reward at the end, whereas in the rat task loss-chasing was programmed to curtail following only 2 extra gambles followed by a punishment (Rogers et al. 2013). This difference may have increased the propensity to loss-chase by the mice as a result of increased behavioural momentum, linked to the fact that increased persistence could result in reward, or as an effort to overcome the potentially greater number of negative outcomes (represented as accumulating delay to the next available reward) present in our mouse task (Nevin 2002). A further hypothesis is related to the involvement of 5-HT mechanisms in mediating feeding (Palacios et al. 2017), where it could be that increased loss-chasing reflected a drive to seek food by continued gambling. Although 8-OH-DPAT treatment increases feeding in non-food deprived rodents (Shepherd and Rogers 1990; Ebenezer and Surujbally 2007), it may lead to decreased or no changes in consumption of palatable foodstuffs (e.g. condensed milk rewards, as used in the current study) at doses comparable with those used here (Dourish et al. 1988; Swiergiel & Dunn 2000; Ebenezer and Tite 2003), thus a feeding-related hypothesis for our findings seems unlikely.
There have been suggestions that putative ‘cognition enhancers’ such as modafinil may be of use in pathological gambling (Łabuzek et al. 2014; Pettorruso et al. 2014). We tested the effects of modafinil in our model and found dose-sensitive reductions in both initial gambling choice and subsequent loss-chasing behaviour. In humans, modafinil has complex effects on gambling behaviours, with dissociable effects in problem gamblers that are dependent on high/low impulsivity traits, such as reducing the desire to gamble, the salience of gambling stimuli (such as gambling-related words) and critically, making risky choices (Zack and Poulos 2004; Smart et al. 2013). In the present work, we did not divide our experimental group into subgroups based on measures of individual impulsivity but found consistent effects of modafinil in reducing gambling choices. The psychological substrates of these effects from the data to hand remain unknown but the most prominent and robust effect of modafinil seems to be on general arousal and attention, rather than any specific cognitive process, via cortical mechanisms (Scoriels et al. 2013; Smart et al. 2013). On that basis, it might be speculated that modafinil could impact on multiple facets of loss-chasing behaviour, including, as we demonstrate here, the control of impulses to gamble in the first place to the control of persistent gambling in the face of repeated losses.
The precise neurobiological mechanism(s) of action of modafinil are, at present, also obscure. Modafinil does not appear to act as a monoamine releaser, as is the case for amphetamine-based stimulants, but has diverse effects on multiple neurotransmitter and modulatory systems encompassing, α-adrenoreceptors, GABA, orexin, dopamine and glutamate systems (Ballon and Feifel 2006), many of which have been implicated in gambling, and underlying psychologies mediating risk and decision making (Potenza 2013). For example, noradrenaline and orexins can affect motivation, arousal and decision-making (Chen et al. 2015; Quintero Garzola 2019) and decreased sensitivity of α2-adrenoreceptors has been reported in pathological gamblers (Quintero Garzola 2019), furthermore, clonidine, an α2-adrenoreceptor agonist decreased risky choice by decreasing reward sensitivity responding in a rat risk-taking task (Montes et al. 2015). Infusions of GABAA and GABAB receptor agonists into the prelimbic or infralimbic regions of the frontal cortex affect decision-making in a rat gambling task (Zeeb et al. 2015), and ketamine and MK801 NMDA receptor antagonists, affecting glutamate transmission, also influence decision making in probabilistic discounting tasks (Yates 2019). Clearly, more work is required to specify the precise brain mechanisms mediating the effects of modafinil on gambling behaviours.
In conclusion, we have developed a novel touchscreen assay to examine dissociable aspects of within-session gambling behaviour in mice. Mice demonstrated the expected patterns of behaviour when the odds for winning were altered resembling gambling behaviours seen in humans and other model species. With mice still ahead of rats as the main tractable genetic model for behavioural and neurobiological research (Muñoz-Fuentes et al. 2018), there is still a need for the development of novel methods of evaluating ‘normal’ mouse behaviour as a means to understand atypical patterns of behaviour following genetic or pharmacological manipulation. Thus, developing tasks such as the G/LCT will enable researchers to investigate a range of mechanisms related to pathological gambling (and response control) using mice as a model organism. We probed neurobiological mechanisms using pharmacological manipulations of the serotonergic system, specifically 5-HT2C and 5-HT1A receptors, and the putative cognition enhancer modafinil. The drugs had different effects on gambling behaviours providing evidence consistent with there being different brain mechanisms and systems operating to influence an initial decision to gamble and subsequent decisions to continue gambling in the face of repeated losses. Loss-chasing behaviour is a key component of problem gambling; hence, this work may have utility in modelling the complex psychological and neurobiological underpinnings of pathological gambling.
References
Assié MB, Koek W (2000) [(3)H]-8-OH-DPAT binding in the rat brain Raphe area: involvement of 5-HT1A and non-5-HT1A receptors. Br J Pharmacol 130:1348–1352
Ballon JS, Feifel D (2006) A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry 67:554–566
Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN (2012) Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol Psychiatry 71:749–757
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacol 38:1083–1152
Bateson M, Kacelnik A (1995) Preferences for fixed and variable food sources: variability in amount and delay. J Exp Anal Behav 63:313–329
Breen RB, Zuckerman M (1999) ‘Chasing’ in gambling behaviour: personality and cognitive determinants. Personal Individ Differ 27:1097–1111
Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM (2012) New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacol 62:1191–1203
Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD (2008) Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry 63:293–300
Campbell-Meiklejohn D, Wakeley J, Herbert V, Cook J, Scollo P, Ray MK, Selvaraj S, Passingham RE, Cowen P, Rogers RD (2011) Serotonin and dopamine play complementary roles in gambling to recover losses. Neuropsychopharmacol 36:402–410
Campbell-Meiklejohn D, Simonsen A, Scheel-Kruger J, Wohlert V, Gjerloff T, Frith CD, Rogers RD, Roepstorff A, Møller A (2012) In for a penny, in for a pound: methylphenidate reduces the inhibitory effect of high stakes on persistent risky choice. J Neurosci 32:13032–13038
Chagraoui A, Thibaut F, Skiba M, Thuillez C, Bourin M (2016) 5-HT2C receptors in psychiatric disorders: a review. Prog Neuro-Psychopharmacol Biol Psychiatry 66:120–135
Chase HW, Clark L (2010) Gambling severity predicts midbrain response to near-miss outcomes. J Neurosci 30:6180–6187
Chen Q, de Lecea L, Hu Z, Gao D (2015) The hypocretin/orexin system: an increasingly important role in neuropsychiatry. Med Res Rev 35:152–197
Clark L (2010) Decision-making during gambling: an integration of cognitive and psychobiological approaches. Philos Trans R Soc Lond Ser B Biol Sci 365:319–330
Clark L, Lawrence AJ, Astley-Jones F, Gray N (2009) Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron 61:481–490
Clifton PG, Kennett GA (2006) Monoamine binding receptors as drug targets for the treatment of obesity. CNS Neurol Disord Drug Targets 5:293–312
Conversano C, Marazziti D, Carmassi C, Baldini S, Barnabei G, Dell'Osso L (2012) Pathological gambling: a systematic review of biochemical, neuroimaging, and neuropsychological findings. Harv Rev Psychiatry 20:130–148
Daw ND, Niv Y, Dayan P (2005) Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 8:1704–1711
Dourish CT, Cooper SJ, Gilbert F, Coughlan J, Iversen SD (1988) The 5HT1A agonist 8-OH-DPAT increases consumption of palatable we mash and liquid diets in the rat. Psychopharmacology 94:58–63
Ebenezer IS, Surujbally A (2007) The effects of 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) on food intake in non-deprived C57BL6 mice. Eur J Pharmacol 22:184–188
Ebenezer IS, Tite R (2003) Differential effects of the 5-HT1A agonist gepirone on food intake in rats may depend on their motivation to eat. Methods Find Exp Clin Pharmacol 25:727–731
Fernandes HA, Zanin KA, Patti CL, Wuo-Silva R, Carvalho RC, Fernandes-Santos L, Bittencourt LR, Tufik S, Frussa-Filho R (2013) Inhibitory effects of modafinil on emotional memory in mice. Neuropharmacol 64:365–370
Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA (2007) Opposing effects of 5-HT2A and 5-HT2C receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology 195:223–234
Gainsbury SM (2015) Online gambling addiction: the relationship between internet gambling and disordered gambling. Curr Addict Rep 2:185–193
Grant JE, Odlaug BL, Schreiber LR (2014) Pharmacological treatments in pathological gambling. Br J Clin Pharmacol 77:375–381
Hewitt KN, Lee MD, Dourish CT, Clifton PG (2002) Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav 71:691–700
Hodgins DC, Stea JN, Grant JE (2011) Gambling disorders. Lancet. 378:1874–1884
Humby T, Laird FM, Davies W, Wilkinson LS (1999) Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci 11:2813–2823
Humby T, Wilkinson L, Dawson G (2005) Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr Protoc Neurosci Ch. 8: unit 8.5H
Humby T, Eddy JB, Good MA, Reichelt AC, Wilkinson LS (2013) A novel translational assay of response inhibition and impulsivity: effects of prefrontal cortex lesions, drugs used in ADHD, and serotonin 2C receptor antagonism. Neuropsychopharmacol 38:2150–2159
Kacelnik A, Bateson M (1996) Risky theories - the effects of variance on foraging decisions. Am Zool 36(4):402–434
Kahneman D, Tversky A (1979) Prospect theory: an analysis of decisions under risk. Econometrica 47:263–291
Kahneman D, Tversky A (2000) Choices Values and Frames. Cambridge University Press, Cambridge 848pp
Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP (1997) SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacol 36:609–620
Kim D, Park GY, O’Doherty JP, Lee SW (2019) Task complexity interacts with state-space uncertainty in the arbitration between model-based and model-free learning. Nat Commun 10:5738
Kyngdon A, Dickerson M (1999) An experimental study of the effect of prior alcohol consumption on a simulated gambling activity. Addiction 94:697–707
Łabuzek K, Beil S, Beil-Gawełczyk J, Gabryel B, Franik G, Okopień B (2014) The latest achievements in the pharmacotherapy of gambling disorder. Pharmacol Rep 66:811–820
Lesieur H (1977) The Chase: career of the compulsive gambler, 1st edn. Garden City, Anchor Press/Doubleday
Lesieur HR (1979) The compulsive gambler's spiral of options and involvement. Psychiatry 42:79–87
Montes DR, Stopper CM, Floresco SB (2015) Noradrenergic modulation of risk/reward decision making. Psychopharmacology 232:2681–2696
Muñoz-Fuentes V, Cacheiro P, Meehan TF, Aguilar-Pimentel JA, Brown SDM, Flenniken AM, Flicek P, Galli A, Mashhadi HH, Hrabě de Angelis M, Kim JK, Lloyd KCK, McKerlie C, Morgan H, Murray SA, Nutter LMJ, Reilly PT, Seavitt JR, Seong JK, Simon M, Wardle-Jones H, Mallon AM, Smedley D, Parkinson HE (2018) IMPC consortium. The International Mouse Phenotyping Consortium (IMPC): a functional catalogue of the mammalian genome that informs conservation. Conserv Genet 19:995–1005
Nahata M, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, Iizuka S, Hattori T, Asaka M, Takeda H (2013) Serotonin 2C receptor antagonism ameliorates novelty-induced hypophagia in aged mice. Psychoneuroendocrinology. 38:2051–2064
Nevin JA (2002) Measuring behavioural momentum. Behav Process 57:187–198
O'Connor J, Dickerson M (2003) Impaired control over gambling in gaming machine and off-course gamblers. Addiction 98:53–60
Palacios JM, Pazos A, Hoyer D (2017) A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology 234:1395–1418
Parke A, Harris A, Parke J, Goddard P (2016) Understanding within-session loss-chasing: an experimental investigation of the impact of stake size on cognitive control. J Gambl Stud 32(2):721–735
Paterson NE, Wetzler C, Hackett A, Hanania T (2012) Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. Int J Neuropsychopharmacol 15:1473–1487
Pettorruso M, De Risio L, Martinotti G, Di Nicola M, Ruggeri F, Conte G, Di Giannantonio M, Janiri L (2014) Targeting the glutamatergic system to treat pathological gambling: current evidence and future perspectives. Biomed Res Int 2014:109786
Pompeiano M, Palacios JM, Mengod G (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 23:163–178
Potenza MN (2013) How central is dopamine to pathological gambling or gambling disorder? Front Behav Neurosci 7:206
Potenza MN, Balodis IM, Franco CA, Bullock S, Xu J, Chung T et al (2013) Neurobiological considerations in understanding behavioral treatments for pathological gambling. Psychol Addict Behav 27:380–392
Quintero Garzola GC (2019) Review: brain neurobiology of gambling disorder based on rodent models. Neuropsychiatr Dis Treat 15:1751–1770
Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW (2008) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacol 33:1028–1037
Rogers RD (2011) The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacol 36:114–132
Rogers RD, Wong A, McKinnon C, Winstanley CA (2013) Systemic administration of 8-OH-DPAT and eticlopride, but not SCH23390, alters loss-chasing behavior in the rat. Neuropsychopharmacol 38:1094–1104
Russek EM, Momennejad I, Botvinick MM, Gershman SJ, Daw ND (2017) Predictive representations can link model-based reinforcement learning to model-free mechanisms. PLoS Comput Biol 13:e1005768
Scoriels L, Jones PB, Sahakian BJ (2013) Modafinil effects on cognition and emotion in schizophrenia and its neurochemical modulation in the brain. Neuropharmacol. 64:168–184
Seymour B, Daw ND, Roiser JP, Dayan P, Dolan R (2012) Serotonin selectively modulates reward value in human decision-making. J Neurosci 32:5833–5842
Sharp T, Bramwell SR, Grahame-Smith DG (1989) 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol 96:283–290
Shepherd JK, Rogers RJ (1990) 8-OH-DPAT specifically enhances feeding behaviour in mice: evidence from behavioural competition. Psychopharmacology 101:408–413
Smart K, Desmond RC, Poulos CX, Zack M (2013) Modafinil increases reward salience in a slot machine game in low and high impulsivity pathological gamblers. Neuropharmacol 73:66–74
Stea JN, Hodgins DC (2011) A critical review of treatment approaches for gambling disorders. Curr Drug Abuse Rev 4:67–80
Swiergiel AH, Dunn AJ (2000) Lack of evidence for a role of serotonin in interleukin-1-induced hypophagia. Pharmacol Biochem Behav 65:531–537
van den Bos R, Davies W, Dellu-Hagedorn F, Goudriaan AE, Granon S, Homberg J, Rivalan M, Swendsen J, Adriani W (2013) Cross-species approaches to pathological gambling: a review targeting sex differences, adolescent vulnerability and ecological validity of research tools. Neurosci Biobehav Rev 37:2454–2471
Vickers SP, Dourish CT, Kennett GA (2001) Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacol 41:200–209
Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology 176:376–385
Worhunsky PD, Malison RT, Rogers RD, Potenza MN (2014) Altered neural correlates of reward and loss processing during simulated slot-machine fMRI in pathological gambling and cocaine dependence. Drug Alcohol Depend 145:77–86
Yates JR (2019) Examining the neurochemical underpinnings of animal models of risky choice: methodological and analytic considerations. Exp Clin Psychopharmacol 27:178–201
Zack M, Poulos CX (2004) Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacol 29:195–207
Zeeb FD, Robbins TW, Winstanley CA (2009) Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacol 34:2329–2343
Zeeb FD, Baarendse PJ, Vanderschuren LJ, Winstanley CA (2015) Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology 232:4481–4491
Acknowledgements
We would like to thank Robyn Dowlen for the help in the early development of the task, and Clive Mann and other members of JBIOS, Cardiff University, for expert animal husbandry.
Funding
The work was funded by awards from the UK Medical Research Council (MRC Centre for Neuropsychiatric Genetics and Genomics), MR/L010305/1 and Wellcome Trust (Strategic Award DEFINE), 100202/Z/12/Z.
Author information
Authors and Affiliations
Contributions
The experiments were conceived by TH, RDR, CAW and LSW. TH, GES, CAB, RS and JC performed the behavioural experiments. TH and LSW analysed the data and wrote the paper with RDR, WD and CAW.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1633 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Humby, T., Smith, G.E., Small, R. et al. Effects of 5-HT2C, 5-HT1A receptor challenges and modafinil on the initiation and persistence of gambling behaviours. Psychopharmacology 237, 1745–1756 (2020). https://doi.org/10.1007/s00213-020-05496-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05496-x