Abstract
Rationale
Recently, an increasing number of emergency cases due to a novel ketamine-like drug, methoxetamine (MXE), were reported in several countries. However, very little is known about the neuropsychopharmacological and reinforcing profile of this compound.
Objectives
Our study aims to investigate the effects of MXE on self-administration (SA) behaviour in comparison to ketamine and on dopaminergic transmission.
Methods
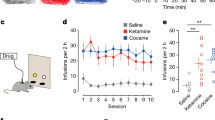
A SA substitution study was performed in male rats trained to intravenously (IV) self-administer ketamine. At responding stability, rats were exposed to sequential phases of MXE substitution at different dosages (starting from 0.5 and then decreasing to 0.25 and 0.125 mg/kg). Standard electrophysiological techniques were used to record changes in firing activities of ventral tegmental area (VTA) dopamine neurons projecting to the nucleus accumbens (NAc) shell after acute injection of cumulative doses of MXE (0.031–0.5 mg/kg IV). Finally, in vivo microdialysis was performed in freely moving rats to evaluate the effect of acute MXE administration (0.125, 0.25 and 0.5 mg/kg IV) on dopamine release in the NAc shell.
Results
MXE 0.125 and 0.25 mg/kg, but not 0.5 mg/kg, substituted for ketamine SA. MXE also induced a dose-dependent stimulation of firing rate (p < 0.0001) and burst firing (p < 0.05) of NAc-projecting VTA dopamine neurons. Consistently, MXE significantly (p < 0.05) increased dopamine extracellular levels in the NAc shell at 0.5 and 0.25 mg/kg with different time onsets, i.e. at 40 and 100 min, respectively.
Conclusions
This study, while confirming the reinforcing effects of MXE, highlights an electrophysiological and neurochemical profile predictive of its addictive properties.






Similar content being viewed by others
References
Adamowicz P, Zuba D (2015) Fatal intoxication with methoxetamine. J Forensic Sci 60:S264–S268. doi:10.1111/1556-4029.12594
Advisory Council on the Misuse of Drugs (ACMD) (2012) Methoxetamine report. https://www.403gov.uk/government/publications/advisory-council-on-the-misuse-of-drugs-acmd-404 methoxetamine-report-2012, 2012 [Accessed July, 2015].
Ator NA, Griffiths RR (2003) Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70:S55–S72
Ault DT, Werling LL (1999) Phencyclidine and dizocilpine modulate dopamine release from rat nucleus accumbens via sigma receptors. Eur J Pharmacol 386:145–153
Balster RL, Bigelow GE (2003) Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend 70:S13–S40
Bassareo V, Di Chiara G (1999) Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89:637–641
Belujon P, Grace AA (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. doi:10.1016/j.biopsych.2014.04.014
Botanas CJ, de la Peña JB, Dela Peña IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH (2015) Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: evidence of its abuse potential. Pharmacol Biochem Behav 133:31–36. doi:10.1016/j.pbb.2015.03.007
Brodie MS, Pesold C, Appel SB (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23:1848–1852
Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ (2002) Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci 116:553–567
Chiamulera C, Armani F, Mutti A, Fattore L (2016) The ketamine analogue methoxetamine generalizes to ketamine discriminative stimulus in rats. Behav Pharmacol 21(2-3 Spec Issue):204–210
Chiappini S, Claridge H, Corkery JM, Goodair C, Loi B, Schifano F (2015) Methoxetamine-related deaths in the UK: an overview. Hum Psychopharmacol 30:244–248. doi:10.1002/hup.2422
Collins GT, Woods JH (2007) Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther 323:599–605. doi:10.1124/jpet.107.123042
Corazza O, Schifano F, Simonato P, Fergus S et al (2012) Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol 27:145–149. doi:10.1002/hup.1242
Corazza O, Assi S, Schifano F (2013) From “Special K” to “Special M”: the evolution of the recreational use of ketamine and methoxetamine. CNS Neurosci Ther 19:454–460. doi:10.1111/cns.12063
CPMP (2000), Position paper on selective serotonin reuptake inhibitors (SSRIs) and dependency/withdrawal reactions, 2000.
CPMP (2002) Note for guidance on clinical investigation of medicinal products in the treatment of depression, 2002.
De Luca MT, Badiani A (2011) Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology 214:549–556. doi:10.1007/s00213-010-2062-x
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278
EMEA (2006) Guideline on the non-clinical investigation of the dependence potential of medicinal products, 2006.
Erhardt S, Engberg G (2002) Increased phasic activity of dopaminergic neurones in the rat ventral tegmental area following pharmacologically elevated levels of endogenous kynurenic acid. Acta Physiol Scand 175:45–53
European Monitoring Centre for Drugs and Drug Addiction-Europol (EMCDDA), Europol Joint Report on a new psychoactive substance: methoxetamine (2-(3-methoxyphenyl)-2-(ethylamino)cyclohexanone), 2014.
Fadda P, Scherma M, Fresu A, Collu M, Fratta W (2003) Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse Oct 50(1):1–6
French ED (1997) delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett 226:159–162
French ED, Ceci A (1990) Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons. Neurosci Lett 119:159–162
Gessa GL, Melis M, Muntoni AL, Diana M (1998) Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341:39–44
Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-1. Identification and characterization. Neuroscience 10:301–315
Green SM, Sherwin TS (2005) Incidence and severity of recovery agitation after ketamine sedation in young adults. Am J Emerg Med 23:142–144
Hancock PJ, Stamford JA (1999) Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth 82(4):603–608. doi:10.1093/bja/82.4.603
Hofer KE, Grager B, Müller DM, Rauber-Lüthy C, Kupferschmidt H, Rentsch KM, Ceschi A (2012) Ketamine-like effects after recreational use of methoxetamine. Ann Emerg Med 60:97–99. doi:10.1016/j.annemergmed.2011.11.018
Huang X, Huang K, Zheng W, Beveridge TJ, Yang S, Li X, Li P, Zhou W, Liu Y (2015) The effects of GSK-3beta blockade on ketamine self-administration and relapse to drug-seeking behavior in rats. Drug Alcohol Depend 147:257–265. doi:10.1016/j.drugalcdep.2014.10.028
Imbert L, Boucher A, Delhome G, Cueto T, Boudinaud M, Maublanc J, Dulaurent S, Descotes J, Lachâtre G, Gaulier JM (2014) Analytical findings of an acute intoxication after inhalation of methoxetamine. J Anal Toxicol 38:410–415. doi:10.1093/jat/bku052
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F (2011) Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A 108:16446–16450. doi:10.1073/pnas.1105418108
Johanson CE, Balster RL (1978) A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull Narc 30:43–54
Jordan S, Chen R, Fernalld R, Johnson J, Regardie K, Kambayashi J, Tadori Y, Kitagawa H, Kikuchi T (2006) In vitro biochemical evidence that the psychotomimetics phencyclidine, ketamine and dizocilpine (MK-801) are inactive at cloned human and rat dopamine D2 receptors. Eur J Pharmacol 540:53–56
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors—implications for models of schizophrenia. Mol Psychiatry 7:837–144
Katz JL (1989) Drugs as reinforcers: pharmacological and behavioural factors. In: Liebman JM, Cooper SJ (eds) The neuropharmacological basis of reward. New York, Oxford, pp 164–213
Kelley AE, Smith-Roe SL, Holahan MR (1997) Response-reinforcement learning is dependent on N-methyl-d-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci U S A 94:12174–12179
Kjellgren A, Jonsson K (2013) Methoxetamine (MXE)—a phenomenological study of experiences induced by a “legal high” from the internet. J Psychoactive Drugs 45:276–286
Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13:177–184
Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M (2012) Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 37:1164–1176. doi:10.1038/npp.2011.302
Linnér L, Endersz H, Ohman D, Bengtsson F, Schalling M, Svensson TH (2001) Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J Pharmacol Exp Ther 297:540–546
Lipski J (1981) Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J Neurosci Methods 4:1–32
Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA Jr (2009) Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther 123:143–150. doi:10.1016/j.pharmthera.2009.02.010
Masuzawa M, Nakao S, Miyamoto E, Yamada M, Murao K, Nishi K, Shingu K (2003) Pentobarbital inhibits ketamine-induced dopamine release in the rat nucleus accumbens: a microdialysis study. Anesth Analg 96:148–152
Morris H, Wallach J (2014) From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs. Drug Test Anal 6:614–632. doi:10.1002/dta
O’Connor EC, Chapman K, Butler P, Mead AN (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938. doi:10.1016/j.neubiorev.2010.10.012
Panin F, Lintas A, Diana M (2014) Nicotine-induced increase of dopaminergic mesoaccumbal neuron activity is prevented by acute restraint stress. In vivo electrophysiology in rats. Eur Neuropsychopharmacol 24:1175–1180. doi:10.1016/j.euroneuro.2014.01.003
Paxinos G, Watson GR (2007) The rat brain in stereotaxic coordinates, 7th edn. Elsevier Academic Press, London
Pontieri FE1, Tanda G, Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Natl Acad Sci 92(26):12304–8
Riegel AC, Zapata A, Shippenberg TS, French ED (2007) The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology 32:1558–1569
Rocha BA, Ward AS, Egilmez Y, Lytle DA, Emmett-Oglesby MW (1996) Tolerance to the discriminative stimulus and reinforcing effects of ketamine. Behav Pharmacol 7:160–168
Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, Iversen L (2013) The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor. PLoS One 8:e59334. doi:10.1371/journal.pone.0059334
Sakamoto S, Nakao S, Masuzawa M, Inada T, Maze M, Franks NP, Shingu K (2006) The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study. Anesth Analg 103:1459–1463
Seeman P, Guan HC (2008) Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse 62:819–828. doi:10.1002/syn.20561
Sein Anand J, Wiergowski M, Barwina M, Kaletha K (2012) Accidental intoxication with high dose of methoxetamine (MXE)—a case report. PrzeglLek 69:609–610
Shields JE, Dargan PI, Wood DM, Puchnarewicz M, Davies S, Waring WS (2012) Methoxetamine associated reversible cerebellar toxicity: three cases with analytical confirmation. Clin Toxicol 50:438–440. doi:10.3109/15563650.2012.683437
Suzuki T, Kato H, Aoki T, Tsuda M, Narita M, Misawa M (2000) Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice. Life Sci 67:383–389
Tan S, Lam WP, Wai MS, Yu WH, Yew DT (2012) Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One 7:e43947. doi:10.1371/journal.pone.0043947
Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050
Tortoriello J, Ortega A, Herrera-Ruíz M, Trujillo J, Reyes-Vázquez C (1998) Galphimine-B modifies electrical activity of ventral tegmental area neurons in rats. Planta Med 64:309–313
Trujillo KA, Zamora JJ, Warmoth KP (2008) Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry 63:178–183. doi:10.1016/j.biopsych.2007.02.014
Ungless MA, Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. doi:10.1016/j.tins.2012.02.003
Van der Kam EL, De Vry J, Tzschentke TM (2007) Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol 18(8):717–24. doi:10.1097/FBP.0b013e3282f18d58
Venniro M, Mutti A, Chiamulera C (2015) Pharmacological and non-pharmacological factors that regulate the acquisition of ketamine self-administration in rats. Psychopharmacology 232:4505–4514. doi:10.1007/s00213-015-4077-9
Wiergowski M, Anand JS, Krzyżanowski M, Jankowski Z (2014) Acute methoxetamine and amphetamine poisoning with fatal outcome: a case report. Int J Occup Med Environ Health 27:683–690. doi:10.2478/s13382-014-0290-8
Wikström M, Thelander G, Dahlgren M, Kronstrand R (2013) An accidental fatal intoxication with methoxetamine. J Anal Toxicol 37:43–46. doi:10.1093/jat/bks086
Wiley JL, Evans RL, Grainger DB, Nicholson KL (2008) Age-dependent differences in sensitivity and sensitization to cannabinoids and ‘club drugs’ in male adolescent and adult rats. Addict Biol 13:277–286
Winstock AR, Lawn W, Deluca P (2015) Borschmann R (2015) Methoxetamine: an early report on the motivations for use, effect profile and prevalence of use in a UK clubbing sample. Drug Alcohol Rev. doi:10.1111/dar.12259
Wood DM, Davies S, Puchnarewicz M, Johnston A, Dargan PI (2012) Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol 68:853–856. doi:10.1007/s00228-011-1199-9
Young AM, Woods JH (1981) Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther 218:720–727
Zawilska JB (2014) Methoxetamine—a novel recreational drug with potent hallucinogenic properties. Toxicol Lett 230:402–407. doi:10.1016/j.toxlet.2014.08.011
Zhang LM, Zhou WW, Ji YJ, Li Y et al (2015) Anxiolytic effects of ketamine in animal models of post traumatic stress disorder. Psychopharmacology 232:663–672. doi:10.1007/s00213-014-3697-9
Acknowledgments
Funded by ‘Joint Project 2012’ from University of Verona, in collaboration with C.N.R. Institute of Neuroscience, Cagliari, and University of Cagliari and a grant from Fondazione Banco di Sardegna 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mutti, A., Aroni, S., Fadda, P. et al. The ketamine-like compound methoxetamine substitutes for ketamine in the self-administration paradigm and enhances mesolimbic dopaminergic transmission. Psychopharmacology 233, 2241–2251 (2016). https://doi.org/10.1007/s00213-016-4275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4275-0