Abstract
Rationale
The abuse of ketamine has been reported to be on the rise over the past 15 years, but its abuse appears to be limited almost exclusively to the context of music and dance settings, indicating a major role of context in modulating its reinforcing effects. We have previously reported that amphetamine, cocaine, and heroin self-administration (SA) in the rat are differentially influenced by the setting in which testing takes place. The aim of the present study is to extend this pre-clinical model to ketamine.
Materials and methods
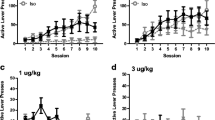
Independent groups of rats with intravenous catheters were given the possibility to self-administer different doses of ketamine (125, 250, and 500 μg/kg per infusion) under two environmental conditions. Some animals were housed in the SA chambers (resident rats) whereas other rats were transported to the SA chambers only for the test sessions (non-resident rats). After training, within-subject dose effect curves (125, 250, 500, and 1,000 μg/kg per infusion) and break-point (during a progressive ratio session) were calculated.
Results
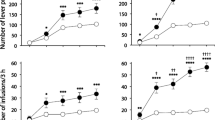
Non-resident rats readily acquired ketamine self-administration. In contrast, resident rats self-administered only the highest dose of ketamine (500 μg/kg), but still four times less than non-resident rats (11.0 ± 6.0 vs 44.4 ± 5.2 infusions during the last training session). No significant differences in break-point were found during the progressive ratio session.
Conclusions
The present study confirms at a preclinical level the importance of setting for ketamine SA and further validates a previously described animal model of drug−environment interaction.




Similar content being viewed by others
References
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anesthetics ketamine and phencyclidine, selectively reduce excitation of central mammalian neurons by N-methyl-aspartate. Br J Pharmacol 79:565–575
Badiani A, Anagnostaras SG, Robinson TE (1995a) The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacol Berl 117:443–4452
Badiani A, Browman KE, Robinson TE (1995b) Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res 674:291–298
Badiani A, Camp DM, Robinson TE (1997) Enduring enhancement of amphetamine sensitization by drug-associated environmental stimuli. J Pharmacol Exp Ther 282:787–794
Badiani A, Oates MM, Robinson TE (2000) Modulation of morphine sensitization in the rat by contextual stimuli. Psychopharmacol Berl 151:273–282
Caprioli D, Paolone G, Celentano M, Testa A, Nencini P, Badiani A (2007a) Environmental modulation of cocaine self-administration in the rat. Psychopharmacol Berl 192:397–406
Caprioli D, Celentano M, Paolone G, Badiani A (2007b) Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psychiatry 31:1639–1653
Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P, Badiani A (2008) Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacol Berl 198:395–404
Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A (2009) Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biol Psychiatry 65:893–899
Celentano M, Caprioli D, Dipasquale P, Cardillo V, Nencini P, Gaetani S, Badiani A (2009) Drug context differently regulates cocaine versus heroin self-administration and cocaine- versus heroin-induced Fos mRNA expression in the rat. Psychopharmacol Berl 204:349–360
Collins RJ, Weeks JR, Cooper MM, Good PI, Russel RR (1984) Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacol Berl 82:6–13
Collins GT, Woods JH (2007) Drug and reinforcement history as determinants of response-maintaining effects quinpirole in the rat. J Pharmacol Exp Ther 323:599–605
Curran HV, Morgan C (2000) Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 95:575–590
Degenhardt L, Dunn M (2008) The epidemiology of GHB and ketamine use in an Australian household survey. Int J Drug Policy 19:311–316
Dworkin S, Smith J (1988) Molecular mechanisms of drug reinforcement—current status. NIDA Res Monogr 90:266–274
Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982) Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacol Berl 78:204–209
Gerrits MA, Van Ree JM (1996) Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res 713:114–124
Hancock PJ, Stamford JA (1999) Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth 82:603–608
Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ (2006) Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci 24:867–875
Jansen KLR (2000) A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs 32:419–433
Joe Laidler KA (2005) The rise of club drugs in a heroin society: the case of Hong Kong. Subst Use Misuse 40:1257–1278
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry 7:837–844
Mendelson LG, Kerchner GA, Kalra V, Zimmerman DM, Leander JD (1984) Phencyclidine receptors in rat brain cortex. Biochem Pharmacol 33:3529–3535
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449
Paolone G, Burdino R, Badiani A (2003) Dissociation in the modulatory effects of environmental novelty on the locomotor, analgesic, and eating response to acute and repeated morphine in the rat. Psychopharmacol Berl 166:146–155
Paolone G, Conversi D, Caprioli D, Bianco PD, Nencini P, Cabib S, Badiani A (2007) Modulatory effect of environmental context and drug history on heroin-induced psychomotor activity and fos protein expression in the rat brain. Neuropsychopharmacology 32:2611–2623
Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacol Berl 84:167–173
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Stinus L, Cador M, Le Moal M (1992) Interaction between endogenous opioids and dopamine within the nucleus accumbens. Ann NY Acad Sci 654:254–273
Trujillo KA, Zamora JJ, Warmoth KP (2007) Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry 63:178–183
Wu LT, Schlenger WE, Galvin DM (2006) Concurrent use of methamphetamine, MDMA, LSD, ketamine, GHB, and flunitrazepam among American Youths. Drug Alcohol Depend 84:102–113
Wolff K, Winstock AR (2006) Ketamine: from medicine to misuse. CNS Drugs 20:199–218
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Luca, M.T., Badiani, A. Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology 214, 549–556 (2011). https://doi.org/10.1007/s00213-010-2062-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2062-x