Abstract
Rationale
Spatial working memory is dependent on the appropriate functioning of the prefrontal cortex (PFC). PFC activity can be modulated by noradrenaline (NA) released by afferent projections from the locus coeruleus. The coreuleo-cortical NA system could therefore be a target for cognitive enhancers of spatial working memory. Of the three classes of NA receptor potentially involved, the α2 and α1 classes seem most significant, though agents targeting these receptors have yielded mixed results. This may be partially due to the use of behavioural assays that do not translate effectively from the laboratory to the clinical setting. Use of a paradigm with improved translational potential may be essential to resolve these discrepancies.
Objectives
The objective of this study was to assess the effects of PFC-infused α2 and α1 adrenergic receptor agonists on spatial working memory performance in the touchscreen continuous trial-unique non-matching to location (cTUNL) task in rats.
Methods
Young male rats were trained in the cTUNL paradigm. Cannulation of the mPFC allowed direct administration of GABA agonists for task validation, and phenylephrine and guanfacine to determine the effects of adrenergic agonists on task performance.
Results
Infusion of muscimol and baclofen resulted in a delay-dependent impairment. Administration of the α2 agonist guanfacine had no effect, whilst infusion of the α1 agonist phenylephrine significantly improved working memory performance.
Conclusions
Spatial working memory as measured in the rat cTUNL task is dependent on the mPFC. Enhancement of noradrenergic signalling enhanced performance in this paradigm, suggesting a significant role for the α1 receptor in this facilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the many cognitive functions for which it is important, the medial prefrontal cortex (mPFC) is perhaps most closely associated with working memory, defined as the ability to transiently maintain and manipulate task-relevant information in a temporary buffer to guide performance (Arnsten et al. 1998; De Luca et al. 2003; Arnsten 2006). Support for a role for the PFC in working memory includes the identification of ‘delay neurons’ in the PFC which fire during the delay between the sample phase and the response (Fuster and Alexander 1971; Kubota and Niki 1971), the observation that these neurons have been shown to represent specific locations within the visual field (Funahashi et al. 1989), and the finding that damage to PFC can lead to delay-dependent impairments in working memory (Dunnett 1990; Seamans et al. 1995; Floresco et al. 1997). Understanding the neurobiological mechanisms by which this structure performs such critical functions is essential both from the perspective of correcting aberrant activity in the context of physical or pathological insult, as well as in enhancing otherwise normal activity.
The catecholamine systems have long been known to play a central role in the regulation of prefrontal-dependent working memory. Catecholamine depletion of both dopamine and NA disrupts working memory performance to a level comparable to that observed following a prefrontal lesion, although performance is unaffected if dopamine depletion is reduced from 87 to 56 % (Brozoski et al. 1979). Coull et al. (1995) found that the mixed α1-α2 agonist clonidine dose-dependently affected CANTAB spatial working memory performance, with significant improvement at a higher dose of clonidine, although which receptor was responsible for this effect is unclear. Subsequently, the α2-R agonist guanfacine, though not clonidine, was found to improve performance on the same task (Jäkälä et al. 1999; see Chamberlain et al. 2006 for a review). It has since been established that pharmacological modulation of prefrontal noradrenergic receptors can bi-directionally shift working memory performance based on the specific receptor sub-type targeted. Indeed, the α1 and α2 noradrenergic receptors (R) have been postulated to have opposing roles within the PFC (Arnsten 1997; Arnsten et al. 1998), with high affinity α2-R activation improving and low-affinity α1-R stimulation impairing PFC function (Arnsten 2000; Robbins and Arnsten 2009). One interpretation of these findings (Arnsten 2000) is that higher levels of NA release, that activate α1-receptors, are associated with stress that impairs spatial working memory according to an inverted U-shaped function.
Functional consequences of noradrenergic receptor engagement can also be influenced by other factors such as baseline NA levels and the level of arousal at the time of drug administration (Arnsten 2000; Birnbaum et al. 2004; Robbins and Arnsten 2009). For example, whilst the α2-R agonists clonidine and guanfacine have both been shown to improve performance on delayed response tasks (Mao et al. 1999; Avery et al. 2000), the majority of studies in which facilitation was detected used rodent or monkey models of age- or experimentally induced catecholamine depletion (e.g. Arnsten and Goldman-Rakic 1985, 1990; Arnsten et al. 1988; Tanila et al. 1996). Similarly, whilst α2-R agonist-induced facilitation can be reversed with an α2-R antagonist (Arnsten and Goldman-Rakic 1985) and α2-R antagonist administration to the primate dorsolateral prefrontal cortex impairs delay-dependent spatial working memory (Li and Mei 1994), the α2-R antagonist atipamezole has been found to facilitate prefrontal-dependent attentional set shifting (Lapiz and Morilak 2006) and attentional measures in the stop signal reaction time task (Bari and Robbins 2013) in the rat.
Pharmacological manipulation of the α1 receptor has yielded equally divergent results, with agonists found to both impair (Arnsten et al. 1999; Mao et al. 1999) and facilitate (Doze et al. 2011) spatial working memory performance. In addition, α1 antagonists have been found to depress baseline or reverse facilitated performance in a number of paradigms, suggesting a potential role for this receptor sub-type in enhancement of prefrontal-dependent cognition (Lapiz and Morilak 2006; Bari and Robbins 2013).
This study investigated the prefrontal adrenergic contribution to delay-dependent spatial working memory performance in the touchscreen cTUNL task (Oomen et al. 2015) in young, healthy rats. The contribution of the mPFC to task performance is unknown. Performance in cTUNL is dependent on the dorsal dentate gyrus of the hippocampus (Oomen et al. 2015), but this effect was found to be independent of the delay condition between trials. The first experiment in the present study, however, confirmed that the mPFC supports delay-dependent performance in the cTUNL task using localised microinfusion of GABAergic agonists, validating a mPFC-dependent working memory deficit. Therefore, cTUNL can be used to dissociate dentate gyrus and mPFC dysfunction, by manipulating the delay condition between trials. In this study, cTUNL was used to assess adrenergic modulation of performance by using the α2 agonist guanfacine and the α1 agonist phenylephrine. Guanfacine was chosen due to its role as a putative cognitive enhancer in the context of normal and pathological ageing, as well as attention deficit hyperactivity disorder (ADHD; Hunt et al. 1985; Arnsten et al. 2007). However, to our knowledge, no studies have yet assessed the efficacy of this compound in non-catecholamine depleted rats. Phenylephrine was selected to further investigate the potential role of the α1 receptor in prefrontal function, with recent evidence indicating a function for this receptor in mediating the cognitive enhancing effects of the atypical psychomotor stimulant modafinil in humans (Winder-Rhodes et al. 2010).
Materials and methods
Subjects
Nine (experiment 1), 18 (experiment 2a) and 14 (experiment 2b) experimentally naïve male Lister Hooded rats (Harlan, UK) weighing a mean of 320 g (range 302–336 g) at the commencement of behavioural training served as subjects. At the time of drug administration, rats were 6–7 months of age. Rats were housed in groups of 4 in a temperature and humidity controlled room, under a 12-h alternating light/dark cycle (lights off, 07:00). Behavioural testing was conducted during the dark phase. Ad libitum access to water was provided throughout. One week following delivery, food was restricted to maintain no less than 85 % of free-feeding weight throughout the entire experiment. All procedures were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986.
Behavioural apparatus
The behavioural apparatus consisted of 12 trapeze-shaped operant chambers [Campden Instruments, UK; 30 cm (height) x 33 cm (length) x 25 cm (width at screen) or x 13 cm (width at magazine)] described elsewhere (Horner et al. 2013). Briefly, chambers consisted of black Perspex walls, a perforated metal grid floor, a transparent lid and a 15-in. touch-sensitive LCD monitor. The touchscreen was covered with a black Perspex mask (35.8 h x 28 w) with 15 response windows (3 rows of 5 locations). Response windows were 3.3 x 3.3 cm in size, spaced 1.5 cm apart (lowest row 1.5 cm from the floor). A food magazine equipped with a LED light and infrared beams was situated opposite the touchscreen. This was connected to an externally located pellet dispenser which provided palatable food reinforcement (45 mg 5-TUL AIN-76A dustless pellets (TestDiet, Indiana, USA)) during cTUNL performance. A click and tone generator and a LED house light were mounted on a shelf above the behavioural arena. Each operant chamber was individually enclosed within a sound attenuating outer box. The behavioural task was controlled by in-house code written in ABET II software (Lafayette Instruments Ltd., USA) running on an Intel E5200 Dual Core Pentium (2.5 GHz) computer. Each chamber was also equipped with infrared beam arrays at the front and rear of the arena to monitor animal activity and an infrared camera to allow visual inspection of behaviour.
cTUNL training
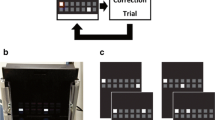
A detailed overview of the cTUNL paradigm is provided elsewhere (Oomen et al. 2015). Briefly, at the start of each session, a single screen location is illuminated with a white square (S+) which must be touched to earn a reward. After a variable delay, the same location (S-) is presented alongside a novel location (S+). The animal must non-match to location by touching the new S+, with the previously correct location becoming the incorrect location on the next trial (see Fig. 1), which continues throughout the rest of the session. Stimuli remain on the screen until the animal has made a response. The spatial separation between the target (S+) and sample (S-) was defined as the number of empty locations between stimuli.
A schematic example of four potential trials of the cTUNL task. The non-matching to location rule is maintained throughout. The novel location (S+) on the previous trial becomes the familiar (S-) location on the next trial. Separation can be manipulated by varying the number of empty squares between stimuli (i.e. trials 2 and 3 are separation 1 trials, whilst trial 4 is a separation 3 trial). Variable delays can be implemented between trials
For both cohorts of rats, the same training protocol was implemented. The pre-training stage (to establish familiarity with the touchscreen chamber and a basic instrumental response) is identical to that of TUNL, which has been described in detail elsewhere (Oomen et al. 2013). For cTUNL task acquisition, rats received 14 days of 1-h training sessions (max 98 trials) with a fixed inter-trial interval (ITI) of 2 s. Rats were trained until individual performance reached a pre-set criterion of 65 % correct for two consecutive sessions (excluding correction trials). Once acquired, rats were rested and re-tested weekly until surgery to avoid overtraining.
Following recovery from surgery, rats were re-baselined on the task with a 2-s fixed ITI/delay (due to the continuous nature of the task, the time period between one trial and the next can be classified as both a delay and an ITI. It will be referred to as a delay in this study). After reaching pre-surgery performance levels, rats were transferred to a schedule in which only separations 1 and 3 (i.e. 1 or 3 empty locations between stimuli only, with no trials presented of separations 0 or 2; see Fig. 1) were used, with a variable delay of 1 and 6 s. The elimination of separation 0 and 2 trials reduced the number of conditions for statistical analysis and was chosen on the basis that the full range of spatial separations is theoretically more relevant to a hippocampal manipulation (Oomen et al. 2015) than the prefrontal focus of this study. The variable delay was introduced to tax working memory. Training continued until a criterion of two consecutive sessions at 65 % correct trial performance (averaged across separations) was reached.
Surgical procedures
All rats received bilateral implantation of 22-gauge indwelling guide cannulas under deep isoflurane-induced anaesthesia. Animals were secured in a stereotaxic frame, and the skull was exposed by an incision along the scalp. This was followed by a craniotomy, after which the guide cannulae (3 mm below pedestal) were implanted at the following stereotaxic coordinates: anteroposterior, 3.2 mm; lateral, 0.75 mm (both from bregma); dorsoventral, −2.0 mm (from dura; Paxinos and Watson 2013). Three jeweller screws and dental cement secured the cannulae. Obdurators sat flush with the guide cannulae and remained in place throughout except during infusions. After surgery, the scalp incision was sutured and post-operative analgesia (metacam; 0.75 mg/0.15 mls.c) and warm saline (37 °C; 0.5 mls.c) were administered. Rats were placed in singularly housed heated cages in a dark room overnight, with ad libitum access to water and food after anaesthetic recovery. Ad libitum food and water access continued for 7 days postoperatively before animals were returned to behavioural testing (with food restriction). Rats were singularly housed for the remainder of the experiment to avoid cannula damage.
Infusion procedure
An experimental timeline is provided in Fig. 2. Rats were habituated to infusions with a session in which the infusion needle was lowered into the cannulae followed a day later by a session in which an infusion of vehicle (at comparable volume and rate to the experimental infusions) was given. Following dose pilot infusions, rats in experiment 1 received bilateral infusions of vehicle or a cocktail of the GABAA receptor agonist muscimol (0.00825 nmol/0.3 μl, Sigma-Aldrich, UK) and the GABAB receptor agonist baclofen (0.0825 nmol/0.3 μl, Sigma-Aldrich, UK). These doses were selected on the basis of pilot infusions in which higher concentrations were found to be sedative (data not shown).
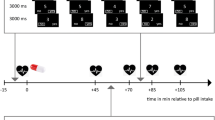
A schematic overview of the experimental procedure adopted for experiment 1 (muscimol + baclofen cocktail vs. vehicle) and experiment 2 (phenylephrine and guanfacine vs. vehicle). The two experiments used two different cohorts of rats. B = one (or more) baseline test sessions (no infusion). Drop = habituation session where the infusion needle is lowered into the cannula, but nothing is infused, followed by behavioural testing. Vehicle infusion = habituation infusion followed by behavioural testing. LS = latin square counterbalanced infusion design with one baseline test session in between each infusion day. For the dose pilots in experiment 1, all rats received two infusions (one vehicle, one muscimol + baclofen cocktail). The difference between LS1 and LS2 in experiment 1 is changes to the behavioural parameters of the task. For the dose pilot in experiment 2, all rats received one infusion only (between subjects design). The data presented is based on LS2 (muscimol + baclofen cocktail vs. vehicle) for experiment 1 (n = 9). For experiment 2a, data is based on LS1 (phenylephrine and guanfacine vs. vehicle, n = 18), and LS2 for experiment 2b (guanfacine vs. vehicle follow up, n = 14). There was a reduction in the n numbers between experiments 2a and b due to cannulae loss
Rats in experiment 2a received bilateral infusions of vehicle (0.5 μl saline, 0.9 %), guanfacine hydrochloride (0.01 μg/0.5 μl or 0.1 μg/0.5 μl in 0.9 % saline, Sigma-Aldrich, UK) or phenylephrine hydrochloride (0.01 μg/0.5 μL or 0.1 μg/0.5 μL in 0.9 % saline, Sigma-Aldrich, UK). Doses for phenylephrine were adopted from (Arnsten et al. 1999). Guanfacine doses were based on Ramos et al. (2006) and increased 100–1000-fold in accordance with the findings of Franowicz and Arnsten (1998) and Avery et al. (2000) which suggested that higher guanfacine doses were required in non-catecholamine depleted animals. Given the limited number of microinfusions possible prior to causing tissue damage at the infusion site, a follow up study (experiment 2b) used a wider range of guanfacine doses (0.001 μg/0.5 μl, 0.0001 μg/0.5 μl, 0.00001 μg/0.5 μl in 0.9 % saline) compared to vehicle (0.9 % saline) but did not extend the dose range for phenylephrine due to the establishment of an effective dose within the initial dose range. Higher doses were not used based on pilot work indicating a sedative effect (data not shown).
For both experiments, infusions were administered in a counterbalanced, within-subjects design to prevent learning effects interfering with potential drug effects. Rats were assigned to counterbalanced groups based on the number of days required to reach criterion (65 % correct overall) on the post-surgery testing schedule (including separations 1 and 3 only, and delays of 1 or 6 s). A latin square design was used to ensure equal numbers of rats received each dose condition on any given infusion day, with no rats receiving each dose condition in the same order of infusions. Rats were mildly restrained, the infusion cannula (28 gauge) lowered into the guides for 1 min pre- and post-infusion. Simultaneous bilateral infusions were administered via two 10-μl Hamilton syringes linked to the cannula via propylene tubing. A precision syringe pump (Harvard Apparatus, Inc.) drove the syringes at a flow rate of 0.15 μL/min (experiment 1) or 0.25 μL/min (experiment 2). Rats were tested within 10 min of infusions. A 48-h washout period was implemented between each infusion, during which one testing session was administered.
Histology
Following completion of behavioural testing, rats were deeply anaesthetised (0.2 ml pentobarbital, i.p.) and transcardially perfused with 0.1 M phosphate-buffered saline followed by neutral-buffered formalin (NBF). Brains were post-fixed in NBF, transferred to 30 % sucrose in dH2O and, once cryoprotected, sliced into 60-μm sections using a cryostat. Sections were stained with Cresyl Violet for placement assessment, verified using a reference atlas (Paxinos and Watson 2013). Whilst it is acknowledged that the mPFC constitutes a range of subregions associated with specific functional and anatomical features such as the division between the medial (medial precentral, dorsal anterior cingulate areas and dorsal part of prelimbic cortex) and the ventral mPFC (ventral prelimbic cortex, infralimbic cortex and medial orbital areas; Heidbreder and Groenewegen 2003), the current study did not aim to differentiate and compare functions across different subregions. Therefore, individual rats were only excluded if placements were outside the mPFC, defined as the combined medial and ventral mPFC (Heidbreder and Groenewegen 2003).
Analysis and statistics
Task accuracy was the main dependent variable, expressed as the percentage of correct responses [(correct trials / total trials) x 100] excluding correction trials. Prior to analysis, all data points were converted to z scores to identify potential outliers, defined as values more than ±2.5 standard deviations away from the mean. As recommended by Field (2013), outliers were removed and replaced with the group mean ±2 standard deviations (i.e. the extremity of the outlier was reduced from ±2.5 to ±2 standard deviations away from the mean). The modified % correct score was then used for statistical analysis. In experiment 1 and 2b, no outliers were detected. In experiment 2a, three outliers were detected and replaced in the guanfacine data set. In the phenylephrine data set, four outliers were detected and replaced. Note that there were 12 individual mean % correct scores associated with each rat (drug [high, low, vehicle] * separation [1 and 3] * delay [1 and 6 s]) in a data set of 18 rats, resulting in a total of 216 mean values, of which a maximum of 4 were identified as outliers within a single data set (the phenylephrine data set).
Latency scores on individual trials exceeding 5 s were excluded, and the median value across the session was used for analysis. Repeated measures ANOVA were used with separation, delay and drug as within-subject factors. Significant interactions were followed up using Sidak’s correction.
Results
Histology
An overview of mPFC cannula locations for experiment 1 (muscimol-baclofen cocktail) is presented in Fig. 3. One rat was excluded from this study due to compromised tissue. A similar overview of cannula locations for experiment 2 (phenylephrine and guanfacine) is presented in Fig. 4. No rats were excluded from this experiment.
Histology for experiment 1 (muscimol-baclofen cocktail). a Schematic of sections of the rat brain (adapted from Paxinos and Watson 2013) displaying the location of placements for rats infused with muscimol-baclofen cocktail. The numbers represent the mm distance from bregma. b Example of Cresyl Violet stained section displaying the location of the guide cannula
Histology for experiment 2 (phenylephrine/guanfacine). a Schematic of sections of the rat brain (adapted from Paxinos and Watson 2013) displaying the location of placements for rats infused with guanfacine and phenylephrine. The numbers represent the mm distance from bregma. b Example of Cresyl Violet stained section displaying the location of the guide cannula
Infusion of muscimol-baclofen into mPFC disrupts cTUNL performance at long delays
High concentrations of muscimol and baclofen (0.033 and 0.33 nmol, respectively) led to sedative effects resulting in a lack of trial completion (data not shown). Lowering the concentration (muscimol = 0.0165 nmol, baclofen = 0.165 nmol) led to a non-specific reduction in percentage correct across long and short delays (data not shown). Using further reduced concentrations of 0.00825 and 0.0825 nmol for muscimol and baclofen, respectively, a drug by delay interaction was identified, in which rats performed significantly worse following infusion of muscimol and baclofen compared to vehicle specifically when the delay was long (F(1,7) = 7.718, p = 0.027; see Fig. 5). A main effect of delay (F(1,7) = 31.888, p = 0.001) and separation (F(1,7) = 40.373, p = 0.0001) was also observed, in which performance was significantly higher when delays were short (p = 0.0001) and separations were large (p = 0.0001). Animals completed all trials within a session, on all infusion conditions, and no differences in latencies were observed (Table 1). Performance during washout period (76.0 ± 1.8) did not differ from that on the vehicle-infused day (73.4 ± 1.8, p > 0.05).
Effect of muscimol and baclofen mPFC microinfusion on performance across delays (1 and 6 s) and separations (1 and 3). Performance on 6-s delay trials was significantly impaired following muscimol and baclofen infusions compared to vehicle. ***p < .001, n = 9. Repeated measures ANOVA with drug, separation and delay as within-subject variables (Sidak’s correction). Error bars ± SEM
Infusion of phenylephrine into mPFC enhances cTUNL performance
Analysis of the five microinfusion conditions [vehicle, low (0.01 μg/0.5 μL) and high (or 0.1 μg/0.5 μL) doses of phenylephrine as well as low (0.01 μg/0.5 μl) and high (0.1 μg/0.5 μl) concentrations of guanfacine] revealed a significant interaction between drug, delay and separation (F(4,68) = 2.622, p = 0.042). This interaction was driven by phenylephrine, as a separate analysis of the two phenylephrine doses and the vehicle infusion also resulted in a significant three-way interaction of drug, delay and separation (F(2,34) = 3.819, p = 0.032). Planned pairwise comparisons revealed that the percent correct scores were significantly improved following the low dose of phenylephrine compared with vehicle on long delay trials when the separation was large (p = 0.039; see Fig. 6). There was no main effect of drug. As expected, a significant main effect of delay (F(1,17) = 52.302, p = 0.0001), separation(F(1,17) = 49.627, p = 0.0001) and a delay and separation interaction (F(1,17) = 20.078, p = 0.0001) was revealed; percent correct performance was significantly higher at 1 versus 6 s delays (p = 0.0001) and at large versus small separation trials (p = 0.0001). The number of trials completed did not differ between infusion conditions (the low phenylephrine dose saw the lowest trial completion rate at 98.2 % completion). No differences in latencies were observed (Table 2).
Effect of phenylephrine mPFC microinfusions on performance across delays (1 and 6 s) and separations (1 and 3). Performance on 1-s delay trials was not influenced by phenylephrine infusions (a). Performance on 6-s delay trials was significantly higher when the separation was large compared to vehicle (b). *p < .05, n = 18. Repeated measures ANOVA with drug, separation and delay as within-subject variables (Sidak’s correction). Error bars ± SEM
Infusions of guanfacine into mPFC failed to affect cTUNL performance
An initial 1-day pilot was undertaken to assess the suitability of the guanfacine doses 0.01 μg/0.5 μl, 0.1 μg/0.5 μl and 1.0 μg/0.5 μl based on the possibility of this compound inducing sedative effects. The highest dose prevented trial completion, and this dose was therefore not used subsequently (data not shown).
Performance during intra-mPFC microinfusions of low (0.01 μg/0.5 μl) and high (0.1 μg/0.5 μl) doses of guanfacine did not differ from vehicle infusions (Fig. 7). There was no significant effect of drug nor an interaction of drug with delay or separation. As expected, a significant main effect of delay (F(1,17) = 55.351, p = 0.001), separation (F(1,17) = 50.576, p = 0.0001) and a delay and separation interaction (F(1,17) = 7.456, p = 0.014) was revealed; percent correct performance was significantly higher at 1 versus 6 s delays (p = 0.0001) and at large versus small separation trials (p = 0.0001). Animals completed all trials within a session, on all infusion conditions, and no differences in latencies were observed (Table 2). Performance during washout period (71.9 ± 1.0) did not differ from that on the vehicle-infused day (72.3 ± 1.8, p > 0.05).
Effect of guanfacine mPFC microinfusions on performance across delays (1 and 6 s) and separations (1 and 3). Performance on neither 1-s delay trials (a) nor 6-s delay trials (b) was affected by guanfacine infusions. N = 18. Repeated measures ANOVA with drug, separation and delay as within-subject variables (Sidak’s correction). Error bars ± SEM
The follow up study with a lower range of guanfacine doses did not reveal any significant effects of microinfusions of guanfacine compared to vehicle (Fig. 8). As before, a main effect of delay (F(1,12) = 124.908, p = 0.0001) and separation (F(1,12) = 43.086, p = 0.0001) was observed, in which performance was higher on short delays (p = 0.0001) and large separations (p = 0.0001). The number of trials completed did not differ between infusion conditions (the low guanfacine dose saw the lowest trial completion rate at 99.0 % completion). No differences in latencies were observed (Table 3). Performance during washout period (74.7 ± 0.8) did not differ from that on the vehicle-infused day (74.9 ± 1.3, p > 0.05).
Effect of follow up doses of guanfacine mPFC microinfusions on performance across delays (1 and 6 s) and separations (1 and 3). Performance on neither 1-s delay trials (a) nor 6-s delay trials (b) was affected by lower guanfacine infusions. N = 14. Repeated measures ANOVA with drug, separation and delay as within-subject variables (Sidak’s correction). Error bars ± SEM
Discussion
The present experiments aimed to test whether the cTUNL task, like TUNL (McAllister et al. 2013), is sensitive to prefrontal dysfunction, as well as to investigate the effects of local adrenergic system modulation on task performance by administration of the α2 receptor agonist guanfacine and the α1 receptor agonist phenylephrine in healthy, young rats. Temporary mPFC inactivation by the GABA agonists muscimol and baclofen resulted in an expected delay-dependent performance deficit, in which performance was unaffected at short delays. Guanfacine did not alter behaviour, whilst administration of low doses of phenylephrine significantly improved performance. This was specific to trials where working memory was taxed through long delays, and stimulus separation was large. On these large separation trials, there may be reduced need for hippocampal-dependent pattern separation processes, as the similarity between the locations represented is low (Talpos et al. 2010; McAllister et al. 2013). This perhaps makes some sense given that the hippocampus, and not mPFC, is required when separations are small (and delay is short) on a similar touchscreen task (Talpos et al. 2010; McAllister et al. 2013). It is worth nothing that there were no observed changes in latencies to respond or collect rewards following any manipulation nor was trial completion affected. In addition, performance was never altered when working memory was not taxed (1 s delay condition), demonstrating normal knowledge of the non-matching rule, as well as a lack of gross motor or motivational changes influencing task performance.
The current study is, to our knowledge, the first demonstration of a cognitive enhancing effect of phenylephrine. This finding contrasts with that of Arnsten et al. (1999) in which mPFC administration resulted in an impairment on delayed alternation performance in the T-maze in young rats. However, the delay was not manipulated within each animal in Arnsten et al. (1999), but rather maintained at an individual length for each rat in order to achieve a uniform performance level of 80 %. These discordant results may therefore be due to the differential taxing of working memory in the two studies. Our finding of a putative cognitive enhancement via engagement of α1 receptors is consistent with the dependence of some of the cognitive enhancing effects of modafinil on this sub-type (Duteil et al. 1990; Lin et al. 1992; Stone et al. 2002). It is particularly notable that the phenylephrine-mediated effect in this study was detected in the most challenging delay condition as administration of the α1 antagonist prazosin reversed the cognitive enhancing effects of modafinil on the One-Touch Stockings of Cambridge planning task only at the most challenging task manipulations. This is suggestive of a role for the α1 receptors in the mediation of enhanced cognitive function (Winder-Rhodes et al. 2010) under relatively specific conditions of cognitive demand. Such findings are probably also relevant to recent findings of improved CANTAB spatial working memory performance following modafinil in healthy volunteers (Müller et al. 2013) and patients with schizophrenia (Scoriels et al. 2012).
Optimal levels of PFC NA enhance working memory via persistent delay-related neuronal firing (Fuster and Alexander 1973; Funahashi et al. 1989; Courtney et al. 1998; Zarahn et al. 1999; Postle et al. 2000). A recent set of experiments found that NA evoked persistent neuronal firing in the PFC through α1 receptors when examining NA effects on pyramidal neurons using combined patch clamp recordings and optogenetics in acute brain slices. Phenylephrine application induced long-lasting, persistent PFC pyramidal neuronal firing, whilst the application of prazosin blocked the NA-evoked persistent response. In contrast, the α2 agonist clonidine did not induce long-lasting, persistent PFC pyramidal neuronal firing, whilst the α2 antagonist yohimbine partially blocked NA-evoked persistent firing (Zhang et al. 2013). Clearly, there is a need for further investigation of the conditions under which stimulation of α1 receptors is cognitively beneficial and the extent to which this can be demonstrated across species using translational tasks assessing prefrontal function.
The lack of a cognitive enhancing effect of guanfacine in this study may appear surprising given the status of this compound as a putative cognitive enhancer. However, the baseline level of NE signalling in the young, healthy animals used in these experiments may account for this discrepancy. Specifically, whilst mostly beneficial effects of guanfacine administration have been reported in aged species with naturally occurring catecholamine depletion, less beneficial effects have been demonstrated in young animals with intact catecholamine systems. For example, in aged monkeys, systemic and centrally administered guanfacine (Arnsten et al. 1988; Arnsten and Goldman-Rakic 1990; Rämä et al. 1996, Arnsten 1997), medetomidine (Rämä et al. 1996) and clonidine (Arnsten and Goldman-Rakic 1990) have been demonstrated to improve spatial working memory task performance. Similarly, systemic administration of medetomidine (Carlson et al. 1992) and microinfusions of guanfacine (Ramos et al. 2006) or medetomidine (Tanila et al. 1996) into the prelimbic cortex of aged rats enhanced spatial working memory performance (although see Sirviö et al. 1992 and Decamp et al. 2011 for a lack of enhancement in aged rats and monkeys respectively). However, the same improvements have not been demonstrated in young adult rats (Carlson et al. 1992; Tanila et al. 1996).
In the context of younger animals, sensitivity to enhancement through systemic administration of clonidine has been achieved in monkeys following catecholamine depletion either specific to the PFC (Arnsten and Goldman-Rakic 1985) or more globally (Cai et al. 1993). In the absence of lowered baseline NA firing, infusions of guanfacine into dorsolateral PFC in young healthy monkeys (Mao et al. 1999) and guanfacine in human volunteers (Jäkälä et al. 1999; Swartz et al. 2008) facilitates spatial working memory performance. However, in cases where cognitive enhancement has been demonstrated via systemic α2 agonists, higher doses than those used with aged or catecholamine depleted animals are required in order for enhancement to be observed (Jackson and Buccafusco 1991; Franowicz and Arnsten 1998; Franowicz and Arnsten 1999; Li et al. 1999; Avery et al. 2000). Some studies have reported no effects of the α2 agonist medetomidine in young control rats, whilst reporting beneficial effects with aged rats in the same experiment; such findings emphasise the importance of age-related catecholamine depletion in the effectiveness of α2 agonism (Carlson et al. 1992; Tanila et al. 1996). If α2 agonist-induced facilitation of performance is related to the chronic central monoaminergic and cholinergic depletion in aged rats, and thus an unregulated sensitivity at PFC α2 receptor sites in the aged species (Luine and Hearns 1990; Luine et al. 1990), it is possible that an enhancement in performance of rats on cTUNL could be observed under more challenging task parameters (such as an increase in the delay), or through the use of natural or experimentally induced catecholamine depletion.
As with the previous report of mPFC infused phenylephrine-driven impairments in rats (Arnsten et al. 1999), the only study in which guanfacine has previously been infused into the (aged) rat mPFC and yielded a cognitive enhancement also assessed behaviour using the T-maze (Ramos et al. 2006). The difference in the tasks used in these and the current study could be a significant contributor to the discordant findings. The restriction of only two locations within the T-maze may promote the use of idiothethic strategies rather than spatial strategies to solve the task, which may reduce the demand on spatial working memory. In contrast, the cTUNL paradigm utilises 15 potential response locations, minimising the utility of alternative and/or mediating strategies to reduce working memory load during the delay. It is possible that the T-maze and the cTUNL task differ in their sensitivity to prefrontally mediated working memory processes under certain conditions, potentially resulting in a different functional response to noradrenergic receptor engagement.
In summary, this study presents the first demonstration of a cognitive enhancing effect of phenylephrine on spatial working memory and highlights the potential for targeting the α1 receptor for facilitation of performance in the context of a young and healthy prefrontal cortex. This was achieved using a novel touchscreen paradigm for measuring spatial working memory in rodents, validated as sensitive to prefrontal inactivation in a delay-dependent manner, and thus illustrating the capacity of the task to detect both facilitation and impairment of prefrontally mediated cognition.
References
Arnsten AF (1997) Catecholamine regulation of the prefrontal cortex. J Psychopharmacol Oxf Engl 11:151–162
Arnsten AF (2000) Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast 7:133–146. doi:10.1155/NP.2000.133
Arnsten AFT (2006) Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 67(Suppl 8):7–12
Arnsten AF, Goldman-Rakic PS (1985) Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science 230:1273–1276
Arnsten AF, Goldman-Rakic PS (1990) Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol Aging 11:583–590
Arnsten AF, Jentsch JD (1997) The alpha-1 adrenergic agonist, cirazoline, impairs spatial working memory performance in aged monkeys. Pharmacol Biochem Behav 58:55–59
Arnsten AF, Cai JX, Goldman-Rakic PS (1988) The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci Off J Soc Neurosci 8:4287–4298
Arnsten AF, Steere JC, Jentsch DJ, Li BM (1998) Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2-adrenergic receptors. Adv Pharmacol San Diego Calif 42:764–767
Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM (1999) Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry 45:26–31
Arnsten AF, Scahill L, Findling RL (2007) alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol 17:393–406. doi:10.1089/cap.2006.0098
Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF (2000) The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 23:240–249. doi:10.1016/S0893-133X(00)00111-1
Bari A, Robbins TW (2013) Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: possible relevance to ADHD. Psychopharmacology (Berl) 230(1):89–111
Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ et al (2004) Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 306:882–884. doi:10.1126/science.1100021
Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–32
Cai JX, Ma YY, Xu L, Hu XT (1993) Reserpine impairs spatial working memory performance in monkeys: reversal by the alpha 2-adrenergic agonist clonidine. Brain Res 614:191–196
Carlson S, Tanila H, Rämä P, Mecke E, Pertovaara A (1992) Effects of medetomidine, an alpha-2 adrenoceptor agonist, and atipamezole, an alpha-2 antagonist, on spatial memory performance in adult and aged rats. Behav Neural Biol 58:113–119
Chamberlain SR, Müller U, Blackwell AD, Robbins TW, Sahakian BJ (2006) Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology 188:397–407
Coull JT, Middleton HC, Robbins TW, Sahakian BJ (1995) Contrasting effects of clonidine and diazepam on tests of working memory and planning. Psychopharmacology 120:311–321
Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV (1998) An area specialized for spatial working memory in human frontal cortex. Science 279:1347–1351
De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K et al (2003) Normative data from the CANTAB. I: development of executive function over the lifespan. IJ Clin Exp Neuropsychol 25:242–254. doi:10.1076/jcen.25.2.242.13639
Decamp E, Clark K, Schneider JS (2011) Effects of the alpha-2 adrenoceptor agonist guanfacine on attention and working memory in aged non-human primates. Eur J Neurosci 34:1018–1022. doi:10.1111/j.1460-9568.2011.07815.x
Doze VA, Papay RS, Goldenstein BL, Gupta MK, Collette KM, Nelson BW et al (2011) Long-term α1A-adrenergic receptor stimulation improves synaptic plasticity, cognitive function, mood, and longevity. Mol Pharmacol 80(4):747–758
Dunnett SB (1990) Role of the prefrontal cortex and striatal output systems in short-term memory deficits associated with aging, basal forebrain lesions, and cholinergic-rich grafts. Can J Psychol 44:210–232
Duteil J, Rambert FA, Pessonnier J, Hermant JF, Gombert R, Assous E (1990) Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol 180:49–58
Field A (2013) Discovering statistics using SPSS, 3rd edn. SAGE Publications Ltd, London
Floresco SB, Seamans JK, Phillips AG (1997) Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17(5):1880–1890
Franowicz JS, Arnsten AF (1998) The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 136:8–14
Franowicz JS, Arnsten AFT (1999) Treatment with the noradrenergic alpha-2 agonist Clonidine, but not Diazepam, improves spatial working memory in normal young rhesus monkeys. Neuropsychopharmacology 21:611–621
Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol 61:331–349
Fuster JM, Alexander GE (1971) Neuron activity related to short-term memory. Science 173:652–654
Fuster JM, Alexander GE (1973) Firing changes in cells of the nucleus medialis dorsalis associated with delayed response behavior. Brain Res 61:79–91
Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR et al (2013) The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc 8:1961–1984
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci & Biobeh Rev 27:555–579
Hunt RD, Minderaa RB, Cohen DJ (1985) Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Psychiatry 24:617–629
Jackson WJ, Buccafusco JJ (1991) Clonidine enhances delayed matching-to-sample performance by young and aged monkeys. Pharmacology Biochemistry and Behavior 39(1):79–84
Jäkälä P, Riekkinen M, Sirviö J, Koivisto E, Kejonen K, Vanhanen M et al (1999) Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology 20:460–470
Kubota K, Niki H (1971) Prefrontal cortical unit activity and delayed alternation in monkeys. J Neurophysiol 34:337–347
Lapiz MDS, Morilak DA (2006) Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137(3):1039–1049
Li BM, Mei ZT (1994) Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol 62(2):134–139
Li BM, Mao ZM, Wang M, Mei ZT (1999) Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 21:601–610. doi:10.1016/S0893-133X(99)00070-6
Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M (1992) Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res 591:319–326
Luine V, Hearns M (1990) Spatial memory deficits in aged rats: contributions of the cholinergic system assessed by ChAT. Brain Res 523:321–324
Luine V, Bowling D, Hearns M (1990) Spatial memory deficits in aged rats: contributions of monoaminergic systems. Brain Res 537:271–278
Mao ZM, Arnsten AF, Li BM (1999) Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry 46:1259–1265
McAllister KAL, Saksida LM, Bussey TJ (2013) Dissociation between memory retention across a delay and pattern separation following medial prefrontal cortex lesions in the touchscreen TUNL task. Neurobiol Learn Mem 101:120–126. doi:10.1016/j.nlm.2013.01.010
Müller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ, Müller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ (2013) Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology 64(1):490–495
Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ et al (2013) The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc 8:2006–2021
Oomen CA, Hvoslef-Eide M, Kofink D, Preusser F, Mar AC, Saksida LM et al (2015) A novel 2- and 3-choice touchscreen-based continuous trial-unique nonmatching-to-location task (cTUNL) sensitive to functional differences between dentate gyrus and CA3 subregions of the hippocampus. Psychopharmacology (Berl). doi:10.1007/s00213-015-4019-6
Paxinos G, Watson C (2013) The rat brain in stereotaxic coordinates, 7th edn. Elsevier Academic Press, London
Postle BR, Berger JS, Taich AM, D’Esposito M (2000) Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci 12(Suppl 2):2–14. doi:10.1162/089892900564028
Rämä P, Linnankoski I, Tanila H, Pertovaara A, Carlson S (1996) Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav 55:415–422
Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AF (2006) Alpha2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn Mem Cold Spring Harb N 13:770–776. doi:10.1101/lm.298006
Robbins TW, Arnsten AFT (2009) The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32:267–287. doi:10.1146/annurev.neuro.051508.135535
Scoriels L, Barnett JH, Soma PK, Sahakian BJ, Jones PB (2012) Effects of modafinil on cognitive functions in first episode psychosis. Psychopharmacology 220(2):249–258
Seamans JK, Floresco SB, Phillips AG (1995) Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Beh Neurosci 109(6):1063
Sirviö J, Harju M, Riekkinen P Jr., Haapalinna A, Riekkinen PJ (1992) Comparative effects of alpha-2 receptor agents and THA on the performance of adult and aged rats in the delayed non-matching to position task. Psychopharmacology (Berl) 109:127–133
Stone EA, Cotecchia S, Lin Y, Quartermain D (2002) Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synap N Y N 46:269–270. doi:10.1002/syn.10127
Swartz BE, McDonald CR, Patel A, Torgersen D (2008) The effects of guanfacine on working memory performance in patients with localization-related epilepsy and healthy controls. Clin Neuropharmacol 31:251–260. doi:10.1097/WNF.0b013e3181633461
Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ (2010) Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem 94:341–352. doi:10.1016/j.nlm.2010.07.006
Tanila H, Rämä P, Carlson S (1996) The effects of prefrontal intracortical microinjections of an alpha-2 agonist, alpha-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Res Bull 40:117–119
Winder-Rhodes SE, Chamberlain SR, Idris MI, Robbins TW, Sahakian BJ, Müller U (2010) Effects of modafinil and prazosin on cognitive and physiological functions in healthy volunteers. J Psychopharmacol Oxf Engl 24:1649–1657. doi:10.1177/0269881109105899
Zarahn E, Aguirre GK, D’Esposito M (1999) Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Res Cogn Brain Res 7:255–268
Zhang Z, Cordeiro Matos S, Jego S, Adamantidis A, Séguéla P (2013) Norepinephrine drives persistent activity in prefrontal cortex via synergistic α1 and α2 adrenoceptors. PloS One 8, e66122. doi:10.1371/journal.pone.0066122
Acknowledgments
The work leading to these results has received funding from the Innovative Medicines Initiative Joint Undertaking (IMI) under grant agreement no. 115008. IMI is a public-private partnership between the European Union and the European Federation of Pharmaceutical Industries and Associations. CJH was funded by Wellcome Trust grant 089703/Z/09/Z. The authors thank Friederike Preusser and Natalie Busch for valuable assistance with behavioural testing in experiment 1.
Disclosures
TWR Consults for Cambridge Cognition, E. Lilly, Lundbeck, Teva, Shire Pharmaceuticals, Otsuka. Research grants from Lilly and Lundbeck. Royalties from Cambridge Cognition (CANTAB). TJB and LMS consult for Campden Instruments Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Martha Hvoslef-Eide and C. A. Oomen contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hvoslef-Eide, M., Oomen, C.A., Fisher, B.M. et al. Facilitation of spatial working memory performance following intra-prefrontal cortical administration of the adrenergic alpha1 agonist phenylephrine. Psychopharmacology 232, 4005–4016 (2015). https://doi.org/10.1007/s00213-015-4038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4038-3