Abstract
Rationale
N-Methyl-d-aspartate (NMDA) receptors in the medial prefrontal cortex (mPFC) are involved in opiate reward processing and modulate sub-cortical dopamine (DA) activity. NMDA receptor blockade in the prelimbic (PLC) division of the mPFC strongly potentiates the rewarding behavioural properties of normally sub-reward threshold doses of opiates. However, the possible functional interactions between cortical NMDA and sub-cortical DAergic motivational neural pathways underlying these effects are not understood.
Objective
This study examines how NMDA receptor modulation in the PLC influences opiate reward processing via interactions with sub-cortical DAergic transmission. We further examined whether direct intra-PLC NMDA receptor modulation may activate DA-dependent opiate reward signaling via interactions with the ventral tegmental area (VTA).
Methods
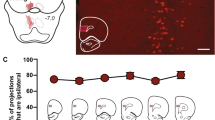
Using an unbiased place conditioning procedure (CPP) in rats, we performed bilateral intra-PLC microinfusions of the competitive NMDA receptor antagonist, (2R)-amino-5-phosphonovaleric acid (AP-5), prior to behavioural morphine place conditioning and challenged the rewarding effects of morphine with DA receptor blockade. We next examined the effects of intra-PLC NMDA receptor blockade on the spontaneous activity patterns of presumptive VTA DA or GABAergic neurons, using single-unit, extracellular in vivo neuronal recordings.
Results
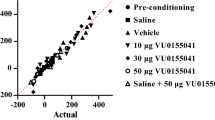
We show that intra-PLC NMDA receptor blockade strongly activates sub-cortical DA neurons within the VTA while inhibiting presumptive non-DA GABAergic neurons. Behaviourally, NMDA receptor blockade activates a DA-dependent opiate reward system, as pharmacological blockade of DA transmission blocked morphine reward only in the presence of intra-PLC NMDA receptor antagonism.
Conclusions
These findings demonstrate a cortical NMDA-mediated mechanism controlling mesolimbic DAergic modulation of opiate reward processing.







Similar content being viewed by others
References
Ahmad T, Lauzon NM, de Jaeger X, Laviolette SR (2013) Cannabinoid transmission in the prelimbic cortex bidirectionally controls opiate reward and aversion signaling through dissociable kappa versus μ-opiate receptor dependent mechanisms. J Neurosci 33:15642–15651
Bishop SF, Lauzon NM, Bechard M, Gholizadeh S, Laviolette SR (2011) NMDA receptor hypofunction in the prelimbic cortex increases sensitivity to the rewarding properties of opiates via dopaminergic and amygdalar substrates. Cereb Cortex 21:68–80
Carlezon WA Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ (2000) Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci 20:RC62
Carr DB, Sesack SR (2000a) GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse 38:114–123
Carr DB, Sesack SR (2000b) Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20:3864–3873
Carr DB, O'Donnell P, Card JP, Sesack SR (1999) Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci 19:11049–11060
De Jaeger X, Bishop SF, Ahmad T, Lyons D, Ng GA, Laviolette SR (2013) The effects of AMPA receptor blockade in the prelimbic cortex on systemic and ventral tegmental area opiate reward sensitivity. Psychopharmacology 225:687–695
Del Arco A, Mora F (1999) Effects of endogenous glutamate on extracellular concentrations of GABA, dopamine, and dopamine metabolites in the prefrontal cortex of the freely moving rat: involvement of NMDA and AMPA/KA receptors. Neurochem Res 24:1027–1035
Del Arco A, Mora F (2002) NMDA and AMPA/kainate glutamatergic agonists increase the extracellular concentrations of GABA in the prefrontal cortex of the freely moving rat: modulation by endogenous dopamine. Brain Res Bull 57:623–630
Del Arco A, Mora F (2008) Prefrontal cortex–nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav 90:226–235
Dolan SL, Bechara A, Nathan PE (2008) Executive dysfunction as a risk marker for substance abuse: the role of impulsive personality traits. Behav Sci Law 26:799–822
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND (2004) Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia 42:1447–1458
Grace AA, Bunney BS (1983) Intracellular and extracellular electrophsyiology of nigral dopaminergic neurons. I. Identification and characterization. Neuroscience 10:301–315
Gysling K, Wang RY (1983) Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 277:119–127
Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB (2006) Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem 98:905–915
Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O'Brien CP, Childress AR (2008) Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry 165:390–394
Laviolette SR, van der Kooy D (2001) GABAA receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci 13:1009–1015
Laviolette SR, van der Kooy D (2003) Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 8:50–59
Laviolette SR, Nader K, van der Kooy D (2002) Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res 129:17–29
Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D (2004) Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci 7:160–169
Lintas A, Chi N, Lauzon NM, Bishop SF, Sun N, Tan H, Laviolette SR (2012) Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. Eur J Neurosci 35:279–290
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706
Luo F, Xi ZX, Wu G, Liu C, Gardner EL, Li SJ (2004) Attenuation of brain response to heroin correlates with the reinstatement of heroin-seeking in rats by fMRI. Neuroimage 22:1328–1335
Margolis EB, Toy B, Himmels P, Morales M, Fields HL (2012) Identification of rat ventral tegmental area GABAergic neurons. PLoS One 7:e42365
Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Nader K, van der Kooy D (1997) Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci 17:383–390
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier, Amsterdam
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053
Sun N, Laviolette SR (2012) Inactivation of the basolateral amygdala during opiate reward learning disinhibits prelimbic cortical neurons and modulates associative memory extinction. Psychopharmacology 222:645–661
Sun W, Rebec GV (2006) Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci 26:8004–8008
Sun N, Chi N, Lauzon N, Bishop S, Tan H, Laviolette SR (2011) Acquisition, extinction, and recall of opiate reward memory are signaled by dynamic neuronal activity patterns in the prefrontal cortex. Cereb Cortex 21:2665–2680
Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR (2009) Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine's rewarding and aversive effects. Neuropharmacology 56:741–751
Tong ZY, Overton PG, Clark D (1996) Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse 22:195–208
Tong ZY, Overton PG, Martinez-Cué C, Clark D (1998) Do non-dopaminergic neurons in the ventral tegmental area play a role in the responses elicited in A10 dopaminergic neurons by electrical stimulation of the prefrontal cortex? Exp Brain Res 118:466–476
Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO (2011) Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci 31:7811–7816
Zhao B, Zhu Y, Wang W, Cui HM, Wang YP, Lai JH (2013) Analysis of variations in the glutamate receptor, N-methyl D-aspartate 2A (GRIN2A) gene reveals their relative importance as genetic susceptibility factors for heroin addiction. PLoS One 8:e70817
Acknowledgments
This work supported by the Canadian Institutes of Health Research (C.I.H.R.) and the National Science and Engineering Council of Canada (N.S.E.R.C.).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, H., Rosen, L.G., Ng, G.A. et al. NMDA Receptor Blockade in the Prelimbic Cortex Activates the Mesolimbic System and Dopamine-Dependent Opiate Reward Signaling. Psychopharmacology 231, 4669–4679 (2014). https://doi.org/10.1007/s00213-014-3616-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3616-0