Abstract
Rationale
Opioid drugs indirectly activate dopamine (DA) neurons in the ventral tegmental area (VTA) through a disinhibition mechanism mediated by mu opioid receptors (MORs) present both on the GABA projection neurons located in the medial tegmental nucleus/tail of the VTA (RMTg/tVTA) and on the VTA GABA interneurons. It is well demonstrated that ethanol, like opioid drugs, provokes VTA DA neuron disinhibition by interacting (through its secondary metabolite, salsolinol) with MORs present in VTA GABA interneurons, but it is not known whether ethanol could disinhibit VTA DA neurons through the MORs present in the RMTg/tVTA.
Objectives
The objective of the present study was to determine whether ethanol, directly microinjected into the tVTA/RMTg, is also able to induce VTA DA neurons disinhibition.
Methods
Disinhibition of VTA DA neurons was indirectly assessed through the analysis of the motor activity of rats. Cannulae were placed into the tVTA/RMTg to perform microinjections of DAMGO (0.13 nmol), ethanol (150 or 300 nmol) or acetaldehyde (250 nmol) in animals pre-treated with either aCSF or the irreversible antagonist of MORs, beta-funaltrexamine (beta-FNA; 2.5 nmol). After injections, spontaneous activity was monitored for 30 min.
Results
Neither ethanol nor acetaldehyde directly administered into the RMTg/tVTA were able to increase the locomotor activity of rats at doses that, in previous studies performed in the posterior VTA, were effective in increasing motor activities. However, microinjections of 0.13 nmol of DAMGO into the tVTA/RMTg significantly increased the locomotor activity of rats. These activating effects were reduced by local pre-treatment of rats with beta-FNA (2.5 nmol).
Conclusions
The tVTA/RMTg does not appear to be a key brain region for the disinhibiting action of ethanol on VTA DA neurons. The absence of dopamine in the tVTA/RMTg extracellular medium, the lack of local ethanol metabolism or both could explain the present results.
Similar content being viewed by others
Introduction
The rostromedial tegmental nucleus (RMTg), also known as the tail of the ventral tegmental area (tVTA), is a midbrain region posterior to the ventral tegmental area (VTA) that sends a dense GABAergic projection to midbrain dopamine (DA) neurons, including VTA and substantia nigra (Jhou et al. 2009; Kaufling et al. 2009). Due to the neuroanatomical position of the tVTA/RMTg and its relation with the lateral habenula and dopamine systems, this brain region has been mainly explored in the context of aversion and avoidance (Sánchez-Catalán et al. 2017; Jhou 2021; Vento et al. 2017; Proulx et al. 2018; Lammel et al. 2012; Stamatakis and Stuber 2012), anxiety and depression (Fu et al. 2019; Elmer et al. 2019; Li et al. 2022; Sun et al. 2020), and prediction error (Hong et al. 2011; Sánchez-Catalán and Barrot 2022). Moreover, it has been related with motor control (Bourdy et al. 2014; Faivre et al. 2020) and pain (Markovic et al. 2021). Finally, the tVTA/RMTg has been studied for its role in the affective state of drugs of abuse, such as opioids (Jhou et al. 2012; Kaufling and Aston-Jones 2015; Matsui and Williams 2011; Steidl et al. 2017; Wasserman et al. 2016), cocaine (Huff and LaLumiere 2015; Jhou et al. 2013; Parrilla-Carrero et al. 2021), and ethanol (Dornellas et al. 2021; Fu et al. 2016a, b; Glover et al. 2016; Sheth et al. 2016).
Recent studies demonstrate that tVTA/RMTg GABA neurons constitute a distinctive group of cells, different both in origin and functions from the VTA GABA interneurons (Lahti et al. 2016; Smith et al. 2019). Despite the differences between VTA GABA neurons and GABA neurons in the tVTA/RMTg, both subsets of neurons share two important characteristics: they show a high expression of mu opioid receptors (MORs) and exert a strong tonic and phasic inhibitory control on VTA DA firing (Jhou et al. 2012; Matsui et al. 2014; Matsui and Williams 2011; Lecca et al. 2011; Jalabert et al. 2011; Fields and Margolis 2015). Indeed, when directly applied in the tVTA/RMTg, opioid drugs, or more precisely, the agonists of MORs, can suppress the inhibitory control on VTA DA firing (Jalabert et al. 2011; Matsui and Williams 2011) in a manner equivalent, although more robust, to that reported by Johnson and North in their seminal paper (Johnson and North 1992). Matsui et al. (2014) compared the magnitude of the opioid-evoked inhibition of VTA DA neuron firing from each GABA afferent, showing that opioids reduce the inhibiting input from tVTA/RMTg more strongly than those from local VTA GABA interneurons. Thus, it has been suggested that the projection from the tVTA/RMTg could play a dominant role in acute opioid disinhibition of VTA DA neurons.
Like opioid drugs, ethanol seems to provoke its rewarding effects by interacting with MORs. To be more precise, it is not ethanol itself that interacts with the MORs but salsolinol, a secondary metabolite of ethanol, which behaves as an agonist of MORs (Xie et al. 2012). Racemic salsolinol can be locally formed in the brain after ethanol administration from the non-enzymatic condensation (Pictet–Spengler reaction) between acetaldehyde (ACH, the primary metabolite of ethanol) and DA (Hipólito et al. 2012), including the VTA (Bassareo et al. 2021). Behavioral and neurochemical data obtained in our laboratory and others, strongly suggest that direct administration of ethanol into the posterior VTA (pVTA) causes an increase in salsolinol levels (Bassareo et al. 2021) and, consequently, an intense activation of the VTA DA neurons (Sánchez-Catalán et al. 2009; Martí-Prats et al. 2010, 2013, 2015; Bassareo et al. 2021). In this sense, the pre-treatment with opioid-antagonists prevents the ability of ethanol to activate VTA DA neurons (Sánchez-Catalán et al. 2009), such as the blockade of the main metabolic systems for ethanol (i.e., catalase) (Marti-Prats et al. 2013; Bassareo et al. 2021). Moreover, direct administration of ACH into the pVTA is able to activate VTA DA neurons (Sánchez-Catalán et al. 2009) and sequestration of ethanol-derived ACH suppresses VTA DA neuron activation (Marti-Prats et al. 2010, 2013; Bassareo et al. 2021). On the other hand, the reduction of the synaptic availability in the VTA of DA impedes salsolinol formation after ethanol administration (Bassareo et al. 2021).
Hence, the ethanol-derived activation of VTA DA neurons seems to depend on (i) the existence of a local metabolism of ethanol that generates ACH and (ii) adequate levels of DA in the brain region considered. Therefore, for ethanol to be able to inhibit the GABAergic input that controls the activity of VTA DA neurons through the MORs, salsolinol must be formed previously and, for this, a double condition must be met: (i) There must be a local metabolism for ethanol that generates ACH and (ii) there must also be a sufficient concentration of DA to react with the ACH locally formed.
Considering the strong influence that the tVTA/RMTg exerts on VTA DA neurons and the high-level expression of MORs in the tVTA/RMTg GABA neurons, one important question arises at this point in the context of brain ethanol actions: could ethanol cause a disinhibition of VTA DA neurons if administered directly into the tVTA/RMTg? In other words, are the two necessary conditions mentioned above met in the tVTA/RMTg so that salsolinol can be formed and, therefore, the GABAergic projection from the tVTA/RMTg inhibited? If we assume that the GABAergic projection from the tVTA/RMTg, as indicated by Matsui’s data (Matsui et al. 2014), plays a crucial role in controlling the activity of VTA DA neurons, this question acquires a special importance. Thus, in the present study, we approach the analysis of this question. Our results suggest that (i) in the tVTA/RMTg the right conditions are not present for the formation of salsolinol after local ethanol administration and (ii) consequently, the tVTA/RMTg is not a critical site for ethanol-derived motor-activating effects in rats.
Materials and methods
Animals
Male Sprague-Dawley rats (280–300 g at the time of surgery) were used for our experiments. They were housed in plastic cages (42 × 27 × 18 cm3) in groups of four to six with controlled humidity and temperature (22°C), a 12:12-h light/dark cycle (on 08:00, off 20:00), and free access to food and water. All the procedures were carried out in strict accordance with the EEC Council Directive 86/609, Spanish laws (RD 1201/2005) and animal protection policies. Experiments were approved by the Animal Care Committee of the Faculty of Pharmacy at the University of Valencia, Spain (protocol codes: 2021/VSC/PEA/0078, approved on 30th March 2021; 2022/VSC/PEA/0037/2, approved on 28th March 2022).
Drugs and chemicals
Ethanol was purchased from Scharlau (Madrid, Spain). ACH and [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO; a selective agonist of the MORs) were purchased from Sigma Chemical Co. (St Louis, MO, USA). β-funaltrexamine (β-FNA; an irreversible antagonist of the MORs) was obtained from Tocris (Bristol, UK). Ethanol and ACH were freshly dissolved in artificial cerebrospinal fluid (aCSF)/ascorbate solution prior to use. The aCSF/ascorbate solution consisted of 120.0 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25.0 mM NaHCO3, 1.2 mM CaCl2, 100 mM D-glucose, and 0.2 mM ascorbate and pH was adjusted to 6.5. Stock solutions of DAMGO and β-FNA were prepared by dissolving the compound in the proper volume of distilled water. These solutions were then kept frozen at −40°C as aliquots until use. Prior to use, aliquots of the stock solutions were conveniently diluted with aCSF/ ascorbate solution to obtain the appropriate concentration. The final pH for the ACH solutions was between 6.4 and 6.6. All the other reagents used were of the highest commercially available grade.
Surgery and post-surgical care
All surgeries were performed under isoflurane anesthesia (1.5–2 minimum alveolar concentration, MAC) and under aseptic conditions. Rats received 2.5 mg/kg of carprofen (s.c) and 0.1% topical lidocaine in the surgical area and in the ears before surgery. Then, animals were placed in a stereotaxic apparatus (Stoelting, USA) and an incision (8–10 mm) was made in the skin above the skull. Three holes were drilled: two for the skull screws and the other for the guide cannulae (Plastics One, USA). Each animal was implanted unilaterally with one 28-gauge guide cannula aimed at 1.0 mm above the tVTA/RMTg. The coordinates relative to bregma and skull surface (Paxinos and Watson 2007) were as follows: A/P −6.9 mm; L ± 1.4 mm; D/V −7.4 mm. Cannulae were angled toward the midline at +6° from the vertical. Cannulae assemblies were secured in place with dental cement. A stainless steel stylet (33-gauge), extending 1.0 mm beyond the tip of the guide cannula, was put in place at the time of surgery and removed at the time of testing. After surgery, rats were housed in individual rectangular plastic cages (42 × 27 × 18 cm3, located side by side in order to prevent the influence of chronic stress on performance due to isolation) with free access to food and water for 7–10 days.
Drug microinjection procedures
All the intra-tVTA/RMTg drug microinjections were carried out with 33-gauge stainless steel injectors, extending 1.0 mm below the tip of the guide cannula. Injectors were attached to a 25 μL Hamilton syringe by using PE-10 tubing. Microinjections were carried out using a syringe pump (Kd Scientific) which was programmed to deliver a total volume of 300 nL (0.15 μl/min) when the pre-treatment (aCSF or β-FNA) was administered, or 200 nL (0.6 μl/min) in the case of the treatment (aCSF, EtOH, ACH or DAMGO). This procedure of administration of the pharmacological agents was identical to that previously used in our previous experiments in the pVTA (Martí-Prats et al. 2010; Sánchez-Catalán et al. 2009). Following the infusion, the injector remained in place for 1 min to allow the diffusion of the drugs, and it was then removed, the stylet was replaced, and the locomotor activity was registered when appropriate. All the injections were carried out in the experimental room.
Handling and test procedure
After 24 h of post-surgical recovery, animals were taken from the colony, brought to the experimental room, and handled for 10 min/day until the experimental day. During this phase, animals became accustomed to the experimenter, the experimental room, and to the injection procedure with a total of four to seven sessions to decrease the activation effects of the manipulations taking place during the injection process, as well as the novelty-activating effects of the testing room. Tests were performed 7–10 days after surgery. On the last habituation day, each animal was also placed in its experimental cage (42 × 27 × 18 cm3) for 30 min to decrease the novelty-activating effects of the testing cage. On the day of the experiment, rats were again taken from the colony room and brought to the experimental room 20–30 min prior to the start of the session, in the same rectangular cages in which the animals were housed. After this initial period, experiments started according to the protocol described in the drug microinjection procedures. Animals were placed in the experimental cage immediately after injection (maximum latency time of 10 s). All the experiments were recorded by a digital video camera for 30 min, and the distance traveled (centimeters) during those 30 min was analyzed for that 30 min using the Raddot program (Universitat de València, Spain). For the handling and test, the experimental room was illuminated with soft white light.
Experiments
Three experiments were conducted:
Experiment 1. Intra-tVTA/RMTg injections of DAMGO and β-FNA+DAMGO
This first experiment had the purpose of verifying, under our experimental conditions, that the local administration of DAMGO into the tVTA/RMTg could cause a MORs-dependent motor activation in the treated animals. We used the same doses of DAMGO and β-FNA that we had previously used in the pVTA (Sánchez-Catalán et al. 2009). We could observe in that paper, that 0.13 nmol DAMGO triggered an intense activation after intra-VTA administration. In the same manner, the β-FNA dose (2.5 nmol) was able to significantly reduce the activation induced by 0.13 nmol DAMGO.
Twenty-eight rats distributed into four subgroups of rats (n=7) were used for the present experiment. The day before the experiment, animals received a pre-treatment with either aCSF or β-FNA (2.5 nmol) depending on the experimental subgroup. On the day of the experiment, rats received a unilateral microinjection of aCSF or DAMGO (0.13 nmol). Thus, the four subgroups formed were: aCSF+aCSF; aCSF+DAMGO; β-FNA+aCSF; β-FNA+DAMGO.
Experiment 2. Intra-tVTA/RMTg injections of ethanol
In our previous papers (Marti-Prats et al. 2010; Sánchez-Catalán et al. 2009), we showed that the intra-pVTA administration of ethanol, at doses of 75 nmol or 150 nmol, increased the motor activity of Wistar rats. In those experiments, injections of ethanol into the pVTA increased the locomotor activity of rats with maximal effects at doses of 150 nmol. These motor-activating effects were significantly reduced by previously administering β-FNA (2.5 nmol) into the pVTA which suggested the involvement of MORs in the motor-activating responses evoked by ethanol. In the present study, we decided to reproduce these results in the tVTA/RMTg using experimental conditions practically identical to those previously used in the pVTA. Therefore, we decided to test the ability of 150 nmol and 300 nmol ethanol directly administered into the tVTA/RMTg to evoke a MORs-dependent motor activation in rats.
Twenty-one Sprague-Dawley rats were randomly assigned to one of the three experimental subgroups (n=7/subgroup). Each animal received two intra-tVTA/RMTg microinjections on consecutive days. On day 1, animals received aCSF, ethanol 150nmol or ethanol 300 nmol depending on the experimental subgroup and motor activity was recorded. On day 2, all animals, regardless of the subgroup to which they belonged, received an intra-tVTA/RMTg microinjection of 0.13 nmol of DAMGO and the locomotor activity of the animals was evaluated once more.
Experiment 3. Intra-tVTA/RMTg injections of ACH
In our previous paper (Sánchez-Catalán et al. 2009), we showed that the intra-pVTA administration of ACH, at doses 250 nmol, increased the motor activity of Wistar rats. In the present study, we decided to test the locomotor-activating effects of this ACH dose after intra-tVTA/RMTg administration. Sixteen Sprague-Dawley rats were randomly assigned to one of the two experimental subgroups (n=7). Each animal received two intra-tVTA/RMTg microinjections on consecutive days. On day 1, animals received aCSF or ACH 250 nmol, depending on the experimental subgroup and motor activity was recorded as indicated above. On day 2, all animals, regardless of the subgroup to which they belonged, received an intra-tVTA/RMTg microinjection of 0.13 nmol of DAMGO and the locomotor activity of the animals was assessed.
Histological validation of the cannula placements
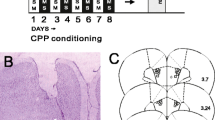
At the end of the experiments, the rats were sacrificed by pentobarbital overdose (90 mg/kg) and the brain was freshly removed and quickly frozen using dry ice. The 40-μm-thick coronal sections of the brain were obtained using a cryostat. Sections were used for the verification of the cannula placements of all the datasets presented in this study. To that end, coronal sections of the tVTA/RMTg were mounted and stained according to the cresyl violet protocol. Then, the location of the cannula tips was carefully examined by a researcher, who was unaware of the experimental condition of the animals, using optical microscopy. A representative photomicrograph is shown in Fig. 1.
Statistical methods
A two-way ANOVA (2 × 2) with interaction was used to analyze the distance traveled by animals across the 30-min sessions in experiment 1. Factors analyzed were “pre-treatment” (aCSF or β-FNA) and “treatment” (aCSF or DAMGO). Post hoc comparisons were made using an adjusted Bonferroni’s test. A mixed two-way ANOVA was used to compare the motor activities obtained in experiments 2 and 3. In both cases, between factor was “treatment on day 1” (with three levels in experiment 2: aCSF, ethanol 150 nmol and ethanol 300 nmol, or two levels in experiment 3: aCSF and ACH 250 nmol) and the within factor was “day of treatment” (with two levels for both experiments: day 1 and day 2). The level of significance was always set at p<0.05. All the analyses were done using SPSS, v. 15.0.
Results
Experiment 1. Intra-tVTA/RMTg injections of DAMGO and β-FNA+DAMGO
Our aim in the present experiment was to confirm that, under our experimental conditions, 0.13 nmol DAMGO can produce a significant motor activation in rats after local intra-tVTA/RMTg administration. It is important to remark that it was previously demonstrated that this dose of DAMGO was able to produce a robust motor activation after intra-pVTA administration (Sánchez-Catalán et al. 2009; Marti- Prats et al. 2010).
Cannula placements in animals used in this experiment are shown in Fig. 2. As can be seen, all animals showed correct placements according to the anatomic characterization of the rat tVTA/RMTg by Smith et al. (2019). Distances traveled by animals used in this experiment are also shown in Fig. 2. As can be observed, only animals pre-treated with aCSF and treated with 0.13 nmol of DAMGO directly into the tVTA/RMTg showed a significant locomotor activation. Two-way ANOVA confirmed these observations. Both the main effects for pre-treatment (F(1,24)=29.975; p<0.001) and treatment (F(1,24)=42.623; p<0.001) were statistically significant. In the same manner, the interaction pre-treatment × treatment was also statistically significant (F(1,24)=23.361; p<0.001). Post hoc comparisons confirmed that the mean distance traveled by animals treated with aCSF/DAMGO differ from acSF/aCSF (p<0.001). Moreover, aCSF/aCSF did not differ between the β-FNA/aCSF group (p=0.654), suggesting that pre-treatment with β-FNA did not modify basal activity of the animals. Moreover, pre-treatment with β-FNA prevented the increase in motor activities of the animals treated with DAMGO (aCSF/DAMGO vs. β-FNA/DAMGO) (p=0.242). Thus, these results confirm that DAMGO, directly administered into the tVTA/RMTg, is able to increase the motor activity of rats, through its interaction with MORs.
Effect of intra-tVTA/RMTg administration of DAMGO on motor activity of rats. Panel A: diagram of coronal sections of the brains of the rats used in experiment 1 showing the placement of the tip of the injection cannulae (aCSF+aCSF (blue), aCSF+DAMGO (red), β-FNA+aCSF (black), β-FNA+DAMGO (violet)). Numbers indicate distance in mm from Bregma (adapted from Paxinos and Watson 2007). Scale bar=1 mm. Panel B: distance traveled (mean±SEM) in 30 min by rats pre-treated with aCSF or β-FNA (2.5 nmol) 1 day before DAMGO (0.13 nmol) or aCSF treatment. A single asterisk (*) indicates significant differences (p<0.05)
Experiment 2. Intra-tVTA/RMTg injections of ethanol
To test if the tVTA/RMTg is, as occurs with the pVTA, a critical region mediating the ethanol activating effects of the VTA DA neurons, we proceeded to administer on day 1 of the experiment, either 150 or 300 nmol of ethanol directly into the tVTA/RMTg. All animals showed correct cannulae placements after histological examination according to the anatomic characterization of the rat tVTA/RMTg by Smith et al. (2019). In order to have pharmacological (in addition to histological) confirmation of the position of the injection cannulae, all the animals used in this experiment received on day 2, a dose of 0.13 nmol of DAMGO which, as we demonstrated in experiment 1, is able to significantly increase the motor activity of the animals.
Results of this experiment are shown in Fig. 3. As can be observed, neither the 150 nmol dose nor the 300 nmol dose of ethanol significantly altered the motor activity of rats on day 1 of the experiment, as compared with activity obtained in the aCSF treated subgroup of animals. The mixed two-way ANOVA with interaction indicated that neither the main effects for the between factor (F(2,18)=1192; p=0.327) nor the interaction ((F(2,18)=1192; p=0.327) were statistically significant. However, the main effects for the within factor were statistically significant (F(1,18)=179,181; p<0.001), being higher on day 2 (DAMGO) than on day 1.
Effect of intra-tVTA/RMTg administration of ethanol on motor activity of rats. Panel A: diagram of coronal sections of the brains of the rats used in experiment 2 indicating the placement of the tip of the injection cannulae (aCSF+aCSF (blue), aCSF+ethanol (150 nmol) (red), aCSF+ethanol (300 nmol) (black)). Numbers indicate distance in mm from Bregma (adapted from Paxinos and Watson 2007). Scale bar=1 mm. Panel B: distance traveled (mean±SEM) in 30 min on day 1 by rats treated with aCSF, ethanol 150 nmol or ethanol 300 nmol (white bars); and on day 2 by rats treated with DAMGO (0.13 nmol) (black bars). A single asterisk (*) indicates significant differences (p<0.05)
Therefore, our results show that ethanol directly administered into the tVTA/RMTg was not able to significantly increase motor activity of the animals. It is unlikely that this finding is due to improper positioning of the injection cannulae since both the histological analysis of the sections (Fig. 3A) and the administration of DAMGO on day 2 of the experiment (administration that led, as we have seen, to highly significant increases in the distance traveled by the animals) suggest that the tips of the cannulae were located in the tVTA/RMTg.
Experiment 3. Intra-tVTA/RMTg injections of ACH
In this experiment, we proceeded to administer on day 1 of the experiment, 250 nmol of ACH directly into the tVTA/RMTg. Previous experiments (Sánchez-Catalán et al. 2009) demonstrated that this dose of ACH is able to produce a significant activation of rats when administered into the pVTA. Histological examination of the brains from animals used in this experiment revealed that all animals showed correct placements according to the anatomic characterization of the rat tVTA/RMTg by Smith et al. (2019). As in experiment 2, in order to have an additional pharmacological confirmation of the position of the injection cannulae, all the animals used in this experiment received on day 2, a dose of 0.13 nmol of DAMGO which, as we demonstrated in experiment 1, is able to significantly increase the motor activity of the animals.
Results of this experiment are shown in Fig. 4. As can be observed, ACH 250 nmol did not significantly alter the motor activity of rats on day 1 of the experiment, as compared with motor activity measured in the aCSF-treated subgroup of animals. The mixed two-way ANOVA with interaction indicated that neither the main effects for the between factor (F(1,12)=0.072; p=0.792) nor the interaction ((F(1,12)=0.070; p=0.797) were statistically significant. However, the main effects for the within factor (“day of treatment”) were statistically significant (F(1,12)=56.421; p<0.001), being higher on day 2 (DAMGO) than on day 1.
Effect of intra-tVTA/RMTg administration of ACH on motor activity of rats. Panel A: diagram of coronal sections of the brains of the rats used in experiment 3 indicating the placement of the tip of the injection cannula (aCSF+aCSF (blue), aCSF+ACH (250 nmol) (red)). Numbers indicate distance in mm from Bregma (adapted from Paxinos and Watson 2007). Scale bar=1 mm. Panel B: white bars: distance traveled (mean±SEM) in 30 min on day 1 by rats treated with aCSF or ACH 250 nmol (white bars); and on day 2 by rats treated with DAMGO (0.13 nmol) (black bars). A single asterisk (*) indicates significant differences (p<0.05)
Our results show that ACH directly administered into the tVTA/RMTg was not able to significantly increase motor activity of the animals. As indicated above for experiment 2, it is unlikely that this finding is due to improper positioning of the injection cannulae since both the histological analysis of the sections (Fig. 4A) and the administration of DAMGO on day 2 of the experiment suggested that the tips of the cannulae were correctly located in the tVTA/RMTg.
Discussion
The results of the present study suggest that the tVTA/RMTg is not a relevant brain region to explain the motor-activating effects of ethanol in the midbrain region through a disinhibition of the VTA DA neurons. As our results show, neither ethanol nor its main metabolite, ACH, locally administered into the tVTA/RMTg at doses that were clearly motor-activating in pVTA (Sánchez-Catalán et al. 2009), were capable of increasing the exploratory motor activity of the rats.
Several research groups have evidenced in the last decade the importance of the tVTA/RMTg and their GABA neurons in controlling the midbrain DA neuron activity (Sanchez-Catalan et al. 2014). At present, it is clear that the activation or inhibition of the tVTA/RMTg potently inhibits or activates, respectively, the activity of midbrain DA neurons (Hong et al. 2011; Kaufling and Aston-Jones 2015; Jalabert et al. 2011; Bourdy et al. 2014; Lecca et al. 2011). As described in the introduction section, the tVTA/RMTg participates in the affective states induced by some drugs of abuse. Specifically, the agonists of MORs mediate an inhibition of the tVTA/RMTg GABA neurons, disinhibiting VTA DA neurons (Jhou et al. 2012; Matsui et al. 2014; Matsui and Williams 2011; Lecca et al. 2011; Jalabert et al. 2011). This indirect activation of the DA system has important functional consequences; for example, this disinhibition helps to explain why rats self-administer MOR agonists into the tVTA/RMTg (Jhou et al. 2012). All the above data demonstrating the existence of this inhibitory control of the tVTA/RMTg over VTA DA neurons has served to review the circuits and the mechanisms involved in the acute and chronic actions of opiate drugs of abuse on the mesolimbic DA neurons (Barrot et al. 2012; Bourdy and Barrot 2012; Kaufling and Aston-Jones 2015). Likewise, the indirect activation of the DA systems also explains why temporary inactivation or injury of the tVTA/RMTg increases exploratory locomotor activity (Lavezzi et al. 2015; Vento et al. 2017). It is noteworthy that the activation of the tVTA/RMTg decreases the consumption and preference for ethanol (Fu et al. 2016a), whereas lesions or pharmacological inhibition (muscimol) of the tVTA/RMTg increase the intake and preference for ethanol (Fu et al. 2016a, b; Sheth et al. 2016).
On the other hand, ethanol is another drug of abuse that seems to exert its acute activating actions on mesolimbic DA neurons through MORs. It has been suggested that actions of ethanol on pVTA DA neurons depend on the interaction of salsolinol (a secondary metabolite of ethanol) with MORs located in the pVTA GABA interneurons (Sánchez-Catalán et al. 2009; Xie et al. 2012; Hipólito et al. 2012; Martí-Prats et al. 2013, 2015; Bassareo et al. 2021). Thus, it seemed of great interest to us to explore whether acute local administration of ethanol into the tVTA/RMTg could, as occurs with classical opiate agonists, trigger a behavioral activation in rats.
Our results show that, contrarily to what has been described for opiates, ethanol directly administered into the tVTA/RMTg was unable to evoke motor activations in the experimental animals. Since, for ethanol to be capable of activating MORs, it must first be biotransformed into salsolinol, it seems logical to explore whether this lack of efficacy to induce motor activation in rats could be due to the lack of salsolinol formation in the tVTA/RMTg after local ethanol administration. The absence of local ethanol metabolism could be one of the explanations for the lack of salsolinol formation: if the enzyme systems necessary to generate ACH do not exist in the tVTA/RMTg, salsolinol cannot be produced. However, there is another possibility that could also help to explain why salsolinol could not be generated after local administration of ethanol: the lack of adequate levels of DA in the tVTA/RMTg. Salsolinol is formed by a non-enzymatic Pictet–Spengler reaction between ACH and DA (Hipolito el al. 2012). Consequently, if DA levels in the tVTA/RMTg are too low or non-existent, the local formation of salsolinol cannot occur, even though the enzyme systems to oxidize ethanol to ACH were present in the tVTA/RMTg.
To explore this intriguing possibility, we decided to administer 250 nmol of ACH directly into the tVTA/RMTg in experiment 3. The rationale of this experiment is obvious: if the lack of effects detected after ethanol administration in experiment 2 was due to the lack of local ethanol metabolism, the direct administration of ACH should be able to overcome this deficiency and cause an increase in the motor activity of the animals as long as tVTA/RMTg DA extracellular levels were adequate to enable salsolinol formation. It is important to remember that, as we demonstrated in previous papers (Sánchez-Catalán et al. 2009), when ACH is directly administered into the pVTA (a brain region with high DA extracellular levels (Adell and Artigas 2004)), animals display a significant motor activation, similar to that observed after ethanol microinjection. Results from our present experiment, however, clearly show that administration of ACH into the tVTA/RMTg does not produce, contrary to what has been observed in the pVTA, any significant change in the motor activity of rats. Obviously, these results, per se, do not support or rule out the existence of ethanol metabolism in the tVTA/RMTg, but they strongly suggest that the lack of motor-activating effects after intra-tVTA/RMTg ethanol administration could be due to the absence of adequate levels of DA in the tVTA/RMTg to generate salsolinol.
Conclusions
It has been suggested that the tVTA/RMTg may be crucial in the context of the mechanisms through which different drugs of abuse, particularly opiates, activate the VTA DA neurons (Barrot et al. 2012; Jhou 2021). Opiates do not need to be biotransformed to interact with their respective receptors, which are abundantly expressed in tVTA/RMTg GABA neurons, making this brain region a key for understanding the mechanism used by these drugs of abuse to activate (disinhibit) midbrain DA neurons. However, ethanol requires both a previous oxidation and condensation of its first metabolite (ACH) with DA to generate the salsolinol molecule, which is ultimately responsible for the interaction with the MORs. According to our results, tVTA/RMTg does not seem to meet the appropriate neurochemical conditions for this to occur.
References
Adell A, Artigas F (2004) The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev 28(4):415–431. https://doi.org/10.1016/j.neubiorev.2004.05.001
Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC (2012) Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci 32(41):14094–14101. https://doi.org/10.1523/JNEUROSCI.3370-12.2012
Bassareo V, Frau R, Maccioni R, Caboni P, Manis C, Peana AT, Migheli R, Porru S, Acquas E (2021) Ethanol-dependent synthesis of salsolinol in the posterior ventral tegmental area as key mechanism of ethanol’s action on mesolimbic dopamine. Front Neurosci 15:675061. https://doi.org/10.3389/fnins.2021.675061
Bourdy R, Barrot M (2012) A new control center for dopaminergic systems: pulling the VTA by the tail. Trends Neurosci 35(11):681–690. https://doi.org/10.1016/j.tins.2012.06.007
Bourdy R, Sánchez-Catalán MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, Sesack SR, Georges F, Barrot M (2014) Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology 39(12):2788–2798. https://doi.org/10.1038/npp.2014.129
Dornellas APS, Burnham NW, Luhn KL, Petruzzi MV, Thiele TE, Navarro M (2021) Activation of locus coeruleus to rostromedial tegmental nucleus (RMTg) noradrenergic pathway blunts binge-like ethanol drinking and induces aversive responses in mice. Neuropharmacology 199:108797. https://doi.org/10.1016/j.neuropharm.2021.108797
Elmer GI, Palacorolla H, Mayo CL, Brown PL, Jhou TC, Brady D, Shepard PD (2019) The rostromedial tegmental nucleus modulates the development of stress-induced helpless behavior. Behav Brain Res 359:950–957. https://doi.org/10.1016/j.bbr.2018.06.014
Faivre F, Sánchez-Catalán MJ, Dovero S, Bido S, Joshi A, Bezard E, Barrot M (2020) Ablation of the tail of the ventral tegmental area compensates symptoms in an experimental model of Parkinson's disease. Neurobiol Dis 139:104818. https://doi.org/10.1016/j.nbd.2020.104818
Fields HL, Margolis EB (2015) Understanding opioid reward. Trends Neurosci 38(4):217–225. https://doi.org/10.1016/j.tins.2015.01.002
Fu R, Zuo W, Gregor D, Li J, Grech D, Ye JH (2016a) Pharmacological manipulation of the rostromedial tegmental nucleus changes voluntary and operant ethanol self-administration in rats. Alcohol Clin Exp Res 40(3):572–582. https://doi.org/10.1111/acer.12974
Fu R, Chen X, Zuo W, Li J, Kang S, Zhou LH, Siegel A, Bekker A, Ye JH (2016b) Ablation of μ opioid receptor-expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol-related behaviors. Neuropharmacology 107:58–67. https://doi.org/10.1016/j.neuropharm.2016.02.027
Fu R, Zuo W, Shiwalkar N, Mei Q, Fan Q, Chen X, Li J, Bekker A, Ye JH (2019) Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus. Neuropsychopharmacology 44(8):1464–1475. https://doi.org/10.1038/s41386-019-0378-8
Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ (2016) Role for the rostromedial tegmental nucleus in signaling the aversive properties of alcohol. Alcohol Clin Exp Res 40(8):1651–1661. https://doi.org/10.1111/acer.13140
Hipólito L, Sánchez-Catalán MJ, Martí-Prats L, Granero L, Polache A (2012) Revisiting the controversial role of salsolinol in the neurobiological effects of ethanol: old and new vistas. Neurosci Biobehav Rev 36(1):362–378. https://doi.org/10.1016/j.neubiorev.2011.07.007
Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O (2011) Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci 31(32):11457–11471. https://doi.org/10.1523/JNEUROSCI.1384-11.2011
Huff ML, LaLumiere RT (2015) The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats. Neuropsychopharmacology 40(4):861–873. https://doi.org/10.1038/npp.2014.260
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F (2011) Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A 108(39):16446–16450. https://doi.org/10.1073/pnas.1105418108
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61(5):786–800. https://doi.org/10.1016/j.neuron.2009.02.001
Jhou TC, Xu SP, Lee MR, Gallen CL, Ikemoto S (2012) Mapping of reinforcing and analgesic effects of the mu opioid agonist endomorphin-1 in the ventral midbrain of the rat. Psychopharmacology 224(2):303–312. https://doi.org/10.1007/s00213-012-2753-6
Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S (2013) Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci 33(17):7501–7512. https://doi.org/10.1523/JNEUROSCI.3634-12.2013
Jhou TC (2021) The rostromedial tegmental (RMTg) “brake” on dopamine and behavior: a decade of progress but also much unfinished work. Neuropharmacology 198:108763. https://doi.org/10.1016/j.neuropharm.2021.108763
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12(2):483–488. https://doi.org/10.1523/JNEUROSCI.12-02-00483.1992
Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M (2009) Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol 513(6):597–621. https://doi.org/10.1002/cne.21983
Kaufling J, Aston-Jones G (2015) Persistent adaptations in afferents to ventral tegmental dopamine neurons after opiate withdrawal. J Neurosci 35(28):10290–10303. https://doi.org/10.1523/JNEUROSCI.0715-15.2015
Lahti L, Haugas M, Tikker L, Airavaara M, Voutilainen MH, Anttila J, Kumar S, Inkinen C, Salminen M, Partanen J (2016) Differentiation and molecular heterogeneity of inhibitory and excitatory neurons associated with midbrain dopaminergic nuclei. Development 143(3):516–529. https://doi.org/10.1242/dev.129957
Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature 491(7423):212–217. https://doi.org/10.1038/nature11527
Lavezzi HN, Parsley KP, Zahm DS (2015) Modulation of locomotor activation by the rostromedial tegmental nucleus. Neuropsychopharmacology 40(3):676–687. https://doi.org/10.1038/npp.2014.223
Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M (2011) Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology 36(3):589–602. https://doi.org/10.1038/npp.2010.190
Li W, Ren Z, Tang Y, Fu Y, Sun S, Ding R, Hou J, Mai Y, Zhan B, Zhu Y, Zuo W, Ye JH, Fu R (2022) Rostromedial tegmental nucleus nociceptin/orphanin FQ (N/OFQ) signaling regulates anxiety- and depression-like behaviors in alcohol withdrawn rats. Neuropsychopharmacology. https://doi.org/10.1038/s41386-022-01482-3 Advance online publication. 10.1038/s41386-022-01482-3
Markovic T, Pedersen CE, Massaly N, Vachez YM, Ruyle B, Murphy CA, Abiraman K, Shin JH, Garcia JJ, Yoon HJ, Alvarez VA, Bruchas MR, Creed MC, Morón JA (2021) Pain induces adaptations in ventral tegmental area dopamine neurons to drive anhedonia-like behavior. Nat Neurosci 24(11):1601–1613. https://doi.org/10.1038/s41593-021-00924-3
Martí-Prats L, Sánchez-Catalán MJ, Hipólito L, Orrico A, Zornoza T, Polache A, Granero L (2010) Systemic administration of D-penicillamine prevents the locomotor activation after intra-VTA ethanol administration in rats. Neurosci Lett 483(2):143–147. https://doi.org/10.1016/j.neulet.2010.07.081
Martí-Prats L, Sánchez-Catalán MJ, Orrico A, Zornoza T, Polache A, Granero L (2013) Opposite motor responses elicited by ethanol in the posterior VTA: the role of acetaldehyde and the non-metabolized fraction of ethanol. Neuropharmacology 72:204–214. https://doi.org/10.1016/j.neuropharm.2013.04.047
Martí-Prats L, Orrico A, Polache A, Granero L (2015) Dual motor responses elicited by ethanol in the posterior VTA: consequences of the blockade of μ-opioid receptors. J Psychopharmacol 29(9):1029–1034. https://doi.org/10.1177/0269881115598337
Matsui A, Williams JT (2011) Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci 31(48):17729–17735. https://doi.org/10.1523/JNEUROSCI.4570-11.2011
Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT (2014) Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82(6):1346–1356. https://doi.org/10.1016/j.neuron.2014.04.030
Parrilla-Carrero J, Eid M, Li H, Chao YS, Jhou TC (2021) Synaptic adaptations at the rostromedial tegmental nucleus underlie individual differences in cocaine avoidance behavior. J Neurosci 41(21):4620–4630. https://doi.org/10.1523/JNEUROSCI.1847-20.2021
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates4th edn. San Diego, CA, Academic Press
Proulx CD, Aronson S, Milivojevic D, Molina C, Loi A, Monk B, Shabel SJ, Malinow R (2018) A neural pathway controlling motivation to exert effort. Proc Natl Acad Sci U S A 115(22):5792–5797. https://doi.org/10.1073/pnas.1801837115
Sánchez-Catalán MJ, Hipólito L, Zornoza T, Polache A, Granero L (2009) Motor stimulant effects of ethanol and acetaldehyde injected into the posterior ventral tegmental area of rats: role of opioid receptors. Psychopharmacology 204(4):641–653. https://doi.org/10.1007/s00213-009-1495-6
Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, Barrot M (2014) The antero-posterior heterogeneity of the ventral tegmental area. J Neurosci 282(12):198–216. https://doi.org/10.1016/j.neuroscience.2014.09.025
Sánchez-Catalán MJ, Faivre F, Yalcin I, Muller MA, Massotte D, Majchrzak M, Barrot M (2017) Response of the tail of the ventral tegmental area to aversive stimuli. Neuropsychopharmacology 42(3):638–648. https://doi.org/10.1038/npp.2016.139
Sánchez-Catalán MJ, Barrot M (2022) Fos response of the tail of the ventral tegmental area to food restriction entails a prediction error processing. Behav Brain Res 425:113826. https://doi.org/10.1016/j.bbr.2022.113826
Sheth C, Furlong TM, Keefe KA, Taha SA (2016) Lesion of the rostromedial tegmental nucleus increases voluntary ethanol consumption and accelerates extinction of ethanol-induced conditioned taste aversion. Psychopharmacology 233(21-22):3737–3749. https://doi.org/10.1007/s00213-016-4406-7
Smith RJ, Vento PJ, Chao YS, Good CH, Jhou TC (2019) Gene expression and neurochemical characterization of the rostromedial tegmental nucleus (RMTg) in rats and mice. Brain Struct Funct 224(1):219–238. https://doi.org/10.1007/s00429-018-1761-7
Stamatakis AM, Stuber GD (2012) Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci 15(8):1105–1107. https://doi.org/10.1038/nn.3145
Steidl S, Wasserman DI, Blaha CD, Yeomans JS (2017) Opioid-induced rewards, locomotion, and dopamine activation: a proposed model for control by mesopontine and rostromedial tegmental neurons. Neurosci Biobehav Rev 83:72–82. https://doi.org/10.1016/j.neubiorev.2017.09.022
Sun Y, Cao J, Xu C, Liu X, Wang Z, Zhao H (2020) Rostromedial tegmental nucleus-substantia nigra pars compacta circuit mediates aversive and despair behavior in mice. Exp Neurol 333:113433. https://doi.org/10.1016/j.expneurol.2020.113433
Vento PJ, Burnham NW, Rowley CS, Jhou TC (2017) Learning from one’s mistakes: a dual role for the rostromedial tegmental nucleus in the encoding and expression of punished reward seeking. Biol Psychiatry 81(12):1041–1049. https://doi.org/10.1016/j.biopsych.2016.10.018
Wasserman DI, Tan JM, Kim JC, Yeomans JS (2016) Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine-induced locomotion. Eur J Neurosci 44(1):1761–1770. https://doi.org/10.1111/ejn.13237
Xie G, Hipólito L, Zuo W, Polache A, Granero L, Krnjevic K, Ye JH (2012) Salsolinol stimulates dopamine neurons in slices of posterior ventral tegmental area indirectly by activating μ-opioid receptors. J Pharmacol Exp Ther 341(1):43–50. https://doi.org/10.1124/jpet.111.186833
Acknowledgements
The authors are indebted to Mr. Ralph Wilk, RSA, TEFL Cambridge who revised the English grammar of the final version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by grants from Conselleria de Educación, Investigación, Cultura y Deporte (Generalitat Valenciana GVA2016-096 and GVA2019-116).
Author information
Authors and Affiliations
Contributions
LG, MJC-C, and TZ designed the study and wrote the protocol; CE-Z and SF-R performed the experiments and analyzed the data; MJC-C, LG and TZ undertook the statistical analysis; LG wrote the first draft of the manuscript; MJS-C contributed on specific parts of the manuscript and provided an overall critical reading; and LG and MJC-C wrote, reviewed, and edited the final version of the manuscript. All authors contributed to and have approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esposito-Zapero, C., Fernández-Rodríguez, S., Sánchez-Catalán, M.J. et al. The rostromedial tegmental nucleus RMTg is not a critical site for ethanol-induced motor activation in rats. Psychopharmacology 240, 2071–2080 (2023). https://doi.org/10.1007/s00213-023-06425-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06425-4