Abstract
Rationale
We have recently reported nicotine-induced stimulation of reelin and glutamic acid decarboxylase 67 (GAD67) mRNA expression levels in the brain of heterozygous reeler mice (HRM), a putative animal model for the study of symptoms relevant to major behavioral disorders.
Objectives
We aimed to evaluate long-term behavioral effects and brain molecular changes as a result of adaptations to nicotine exposure in the developing HRM males.
Methods
Adolescent mice (pnd 37–42) were exposed to oral nicotine (10 mg/l) in a 6-day free-choice drinking schedule. As expected, no differences in total nicotine intake between WT (wild-type) mice and HRM were found.
Results
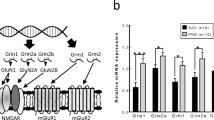
Long-term behavioral effects and brain molecular changes, as a consequence of nicotine exposure during adolescence, were only evidenced in HRM. Indeed, HRM perseverative exploratory behavior and poor cognitive performance were modulated to WT levels by subchronic exposure to nicotine during development. Furthermore, the expected reduction in the expression of mRNA of reelin and GAD67 in behaviorally relevant brain areas of HRM appeared persistently restored by nicotine. For brain-derived neurotrophic factor (BDNF) mRNA expression, no genotype-dependent changes appeared. However, expression levels were increased by previous nicotine in brains from both genotypes. The mRNA encoding for nicotine receptor subunits (α7, β2 and α4) did not differ between genotypes and as a result of previous nicotine exposure.
Conclusion
These findings support the hypothesis of pre-existing vulnerability (based on haploinsufficiency of reelin) to brain and behavioral disorders and regulative short- and long-term effects associated with nicotine modulation.



Similar content being viewed by others
Abbreviations
- GAD67:
-
Glutamic acid decarboxylase
- HRM:
-
Heterozygous reeler mice
- BDNF:
-
Brain-derived neurotrophic factor
- nAChRs:
-
Acetylcholine nicotinic receptors
- DNMT1:
-
DNA methyltransferase 1
References
Adriani W, Laviola G (2004) Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol 15:341–352
Adriani W, Macri S, Pacifici R, Laviola G (2002a) Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology 27:212–224
Adriani W, Macri S, Pacifici R, Laviola G (2002b) Restricted daily access to water and voluntary nicotine oral consumption in mice: methodological issues and individual differences. Behav Brain Res 134:21–30
Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV (2003) Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 23:4712–4716
Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G (2004) Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology 29:869–878
Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE Jr, Jones EG (1995) GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex 5:550–560
Albuquerque EX, Pereira EF, Braga MF, Alkondon M (1998) Contribution of nicotinic receptors to the function of synapses in the central nervous system: the action of choline as a selective agonist of alpha 7 receptors. J Physiol Paris 92:309–316
Albuquerque EX, Pereira EF, Alkondon M, Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120
Arenas E, Akerud P, Wong V, Boylan C, Persson H, Lindsay RM, Altar CA (1996) Effects of BDNF and NT-4/5 on striatonigral neuropeptides or nigral GABA neurons in vivo. Eur J Neurosci 8:1707–1717
Aultman JM, Moghaddam B (2001) Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 153:353–364
Ballmaier M, Zoli M, Leo G, Agnati LF, Spano P (2002) Preferential alterations in the mesolimbic dopamine pathway of heterozygous reeler mice: an emerging animal-based model of schizophrenia. Eur J Neurosci 15:1197–1205
Batel P (2000) Addiction and schizophrenia. Eur Psychiatry 15:115–122
Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M (2007) Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A 104:10164–10169
Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S (2000) Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 23:351–364
Brenhouse HC, Andersen SL (2011) Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev 35:1687–1703
Calamandrei G, Pennazza S, Ricceri L, Valanzano A (1996) Neonatal exposure to anti-nerve growth factor antibodies affects exploratory behavior of developing mice in the hole board. Neurotoxicol Teratol 18:141–146
Chudasama Y, Robbins TW (2006) Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 73:19–38
Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C (2001) Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis 8:723–742
Counotte DS, Goriounova NA, Moretti M, Smoluch MT, Irth H, Clementi F, Schoffelmeer AN, Mansvelder HD, Smit AB, Gotti C, Spijker S (2012) Adolescent nicotine exposure transiently increases high-affinity nicotinic receptors and modulates inhibitory synaptic transmission in rat medial prefrontal cortex. Faseb J 26:1810–1820
Cowansage KK, LeDoux JE, Monfils MH (2010) Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol 3:12–29
Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM (2011) Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology 36:1319–1331
D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719–723
De Filippis B, Ricceri L, Laviola G (2010) Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav 9:213–223
D'Souza MS, Markou A (2012) Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology 62:1564–1573
Fatemi SH, Earle JA, McMenomy T (2000) Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 5(654–63):571
Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ (1992) A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41:31–37
Freedman R, Hall M, Adler LE, Leonard S (1995) Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33
Freedman R, Adams CE, Leonard S (2000) The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat 20:299–306
Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, Calamandrei G, Scarpa S (2008) B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci 37:731–746
Goriounova NA, Mansvelder HD (2012) Nicotine exposure during adolescence leads to short- and long-term changes in spike timing-dependent plasticity in rat prefrontal cortex. J Neurosci 32:10484–10493
Govind AP, Walsh H, Green WN (2012) Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci 32:2227–2238
Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57:1061–1069
Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E (2005) GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 180:191–205
Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P (2010) Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 60:1007–1016
Hughes RN (2004) The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 28:497–505
Ikeda Y, Yahata N, Ito I, Nagano M, Toyota T, Yoshikawa T, Okubo Y, Suzuki H (2008) Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res 101:58–66
Kandel DB, Chen K (2000) Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob Res 2:263–274
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER (2006) Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49:603–615
Kliethermes CL, Crabbe JC (2006) Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacol Biochem Behav 85:57–65
Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nairn AC (2006) Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology (Berl) 189:95–104
Kumari V, Postma P (2005) Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev 29:1021–1034
Kundakovic M, Chen Y, Costa E, Grayson DR (2007) DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharmacol 71:644–653
Lalonde R (2002) The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26:91–104
Laviola G, Marco EM (2011) Passing the knife edge in adolescence: brain pruning and specification of individual lines of development. Neurosci Biobehav Rev 35:1631–1633
Laviola G, Adriani W, Terranova ML, Gerra G (1999) Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev 23:993–1010
Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003) Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27:19–31
Laviola G, Adriani W, Gaudino C, Marino R, Keller F (2006) Paradoxical effects of prenatal acetylcholinesterase blockade on neuro-behavioral development and drug-induced stereotypies in reeler mutant mice. Psychopharmacology (Berl) 187:331–344
Laviola G, Ognibene E, Romano E, Adriani W, Keller F (2009) Gene–environment interaction during early development in the heterozygous reeler mouse: clues for modelling of major neurobehavioral syndromes. Neurosci Biobehav Rev 33:560–572
Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R (2000) Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol 393:237–242
Leonard S, Mexal S, Freedman R (2007) Smoking, genetics and schizophrenia: evidence for self medication. J Dual Diagn 3:43–59
Levin ED, Rezvani AH (2007) Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol 74:1182–1191
Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324
Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E (2001) Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci U S A 98:3477–3482
Lu B, Martinowich K (2008) Cell biology of BDNF and its relevance to schizophrenia. Novartis Found Symp 289: 119–29; discussion 129–35, 193–5
Lv J, Mao C, Zhu L, Zhang H, Pengpeng H, Xu F, Liu Y, Zhang L, Xu Z (2008) The effect of prenatal nicotine on expression of nicotine receptor subunits in the fetal brain. Neurotoxicology 29:722–726
Macri S, Biamonte F, Romano E, Marino R, Keller F, Laviola G (2010) Perseverative responding and neuroanatomical alterations in adult heterozygous reeler mice are mitigated by neonatal estrogen administration. Psychoneuroendocrinology 35:1374–1387
Maloku E, Kadriu B, Zhubi A, Dong E, Pibiri F, Satta R, Guidotti A (2011) Selective alpha4beta2 nicotinic acetylcholine receptor agonists target epigenetic mechanisms in cortical GABAergic neurons. Neuropsychopharmacology 36:1366–1374
Marks MJ, Burch JB, Collins AC (1983) Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226:817–825
Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12:2765–2784
Miao H, Liu C, Bishop K, Gong ZH, Nordberg A, Zhang X (1998) Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. J Neurochem 70:752–762
Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW (2008) Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res 188:178–194
Ochoa EL, Lasalde-Dominicci J (2007) Cognitive deficits in schizophrenia: focus on neuronal nicotinic acetylcholine receptors and smoking. Cell Mol Neurobiol 27:609–639
Ognibene E, Adriani W, Granstrem O, Pieretti S, Laviola G (2007) Impulsivity-anxiety-related behavior and profiles of morphine-induced analgesia in heterozygous reeler mice. Brain Res 1131:173–180
Ognibene E, Adriani W, Caprioli A, Ghirardi O, Ali SF, Aloe L, Laviola G (2008) The effect of early maternal separation on brain derived neurotrophic factor and monoamine levels in adult heterozygous reeler mice. Prog Neuropsychopharmacol Biol Psychiatry 32:1269–1276
Olincy A, Young DA, Freedman R (1997) Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 42:1–5
Pacheco MA, Pastoor TE, Lukas RJ, Wecker L (2001) Characterization of human alpha4beta2 neuronal nicotinic receptors stably expressed in SH-EP1 cells. Neurochem Res 26:683–693
Picciotto MR, Zoli M (2008) Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci 13:492–504
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Podhorna J, Didriksen M (2004) The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res 153:43–54
Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ (2006) Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem 85:228–242
Rapoport JL (2013) Prevention of schizophrenia: an impossible dream? Am J Psychiatry 170:245–247
Ricceri L, De Filippis B, Fuso A, Laviola G (2011) Cholinergic hypofunction in MeCP2-308 mice: beneficial neurobehavioural effects of neonatal choline supplementation. Behav Brain Res 221:623–629
Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, Ernfors P, Ibanez CF (1998) BDNF regulates reelin expression and Cajal–Retzius cell development in the cerebral cortex. Neuron 21:305–315
Romano E, Fuso A, Laviola G (2013) Nicotine restores Wt-like levels of reelin and GAD67 gene expression in brain of heterozygous reeler mice. Neurotox Res 24:205–215
Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, Leonard S, Stevens KE, Freedman R (2013) Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry 170:290–298
Roth TL, Sweatt JD (2011) Epigenetic marking of the BDNF gene by early-life adverse experiences. Horm Behav 59:315–320
Rowell PP, Li M (1997) Dose–response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem 68:1982–1989
Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A (2007) Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry 12:385–397
Saito M, O'Brien D, Kovacs KM, Wang R, Zavadil J, Vadasz C (2005) Nicotine-induced sensitization in mice: changes in locomotor activity and mesencephalic gene expression. Neurochem Res 30:1027–1035
Salinger WL, Ladrow P, Wheeler C (2003) Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci 117:1257–1275
Salin-Pascual RJ, Alcocer-Castillejos NV, Alejo-Galarza G (2003) Nicotine dependence and psychiatric disorders. Rev Invest Clin 55:677–693
Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A (2008) Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci U S A 105:16356–16361
Schneider T, Bizarro L, Asherson PJ, Stolerman IP (2012) Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacology (Berl) 223:401–415
Schwartz RD, Kellar KJ (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220:214–216
Singh A, Potter A, Newhouse P (2004) Nicotinic acetylcholine receptor system and neuropsychiatric disorders. IDrugs 7:1096–1103
Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ (2007a) Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology 32:1082–1097
Slotkin TA, Ryde IT, Seidler FJ (2007b) Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res Bull 74:91–103
Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ (2008) Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res Bull 76:152–165
Spear L (2000a) Modeling adolescent development and alcohol use in animals. Alcohol Res Health 24:115–123
Spear LP (2000b) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463
Takeda H, Tsuji M, Matsumiya T (1998) Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350:21–29
Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H (2002) Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res 110:249–257
Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A (2002) An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci U S A 99:17095–17100
Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C (1999) The phenotypic characteristics of heterozygous reeler mouse. Neuroreport 10:1329–1334
Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E (2006) Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev 30:1065–1077
Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E (2007) Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res 91:51–61
Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE (2003) Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 8:592–610
Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, Weinberger DR, Kleinman JE (2005) Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry 10:637–650
Wilcox RR (1987) New Statistical Procedures for the Social Sciences. Modern Solution To Basic Problems. Hillsdale, New Jersey
Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG (1989) Presynaptic modulation of transmitter release by nicotinic receptors. Prog Brain Res 79:157–163
Woods BT (1998) Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry 155:1661–1670
Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N (2002) Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci 22:7580–7585
Zaheer A, Haas JT, Reyes C, Mathur SN, Yang B, Lim R (2006) GMF-knockout mice are unable to induce brain-derived neurotrophic factor after exercise. Neurochem Res 31:579–584
Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, Guidotti AR, Holden JJ (2002) Reelin gene alleles and susceptibility to autism spectrum disorders. Mol Psychiatry 7:1012–1017
Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, Sershen H, Lajtha A, Smith RC, Guidotti A, Davis JM, Costa E (2009) An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophr Res 111:115–122
Zimmerberg B, Brett MB (1992) Effects of early environmental experience on self-administration of amphetamine and barbital. Psychopharmacology (Berl) 106:474–478
Acknowledgements
We are grateful to Giovanni Dominici for animal care. E.R. is supported by a fellowship from the ERAnet "PrioMedChild", Italian Ministry of Health (P.I. Walter Adriani). The authors are grateful to Dr. Saira Shamsi for critical reading of the manuscript. This research was supported by IRE-IFO (RF2008) "MECP2 phosphorilation and related kinase in Rett syndrome" to GL, E.R. is recipient of a Postdoctoral fellowship under the "NeuroGenMRI" project in the framework of ERAnet "PrioMedChild" Program.
Conflict of interest
We also declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romano, E., De Angelis, F., Ulbrich, L. et al. Nicotine exposure during adolescence: cognitive performance and brain gene expression in adult heterozygous reeler mice. Psychopharmacology 231, 1775–1787 (2014). https://doi.org/10.1007/s00213-013-3388-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3388-y